Structural basis of growth-related gain and age-related loss of bone strength
- PMID: 18556646
- PMCID: PMC2427165
- DOI: 10.1093/rheumatology/ken177
Structural basis of growth-related gain and age-related loss of bone strength
Abstract
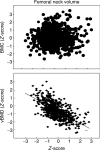
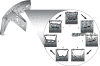
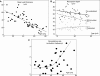
If bone strength was the only requirement of skeleton, it could be achieved with bulk, but bone must also be light. During growth, bone modelling and remodelling optimize strength, by depositing bone where it is needed, and minimize mass, by removing it from where it is not. The population variance in bone traits is established before puberty and the position of an individual's bone size and mass tracks in the percentile of origin. Larger cross-sections have a comparably larger marrow cavity, which results in a lower volumetric BMD (vBMD), thereby avoiding bulk. Excavation of a marrow cavity thus minimizes mass and shifts the cortex radially, increasing rigidity. Smaller cross-sections are assembled by excavating a smaller marrow cavity leaving a relatively thicker cortex producing a higher vBMD, avoiding the fragility of slenderness. Variation in cellular activity around the periosteal and endocortical envelopes fashions the diverse shapes of adjacent cross-sections. Advancing age is associated with a decline in periosteal bone formation, a decline in the volume of bone formed by each basic multicellular unit (BMU), continued resorption by each BMU, and high remodelling after menopause. Bone loss in young adulthood has modest structural and biomechanical consequences because the negative BMU balance is driven by reduced bone formation, remodelling is slow and periosteal apposition continues shifting the thinned cortex radially. But after the menopause, increased remodelling, worsening negative BMU balance and a decline in periosteal apposition accelerate cortical thinning and porosity, trabecular thinning and loss of connectivity. Interstitial bone, unexposed to surface remodelling becomes more densely mineralized, has few osteocytes and greater collagen cross-linking, and accumulates microdamage. These changes produce the material and structural abnormalities responsible for bone fragility.
Keywords: Ageing; Bone; Fragility; Growth; Modelling; Remodelling; Strength.
Figures

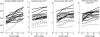

References
-
- Currey JD. Structure and mechanics. Princeton, NJ: Princeton University Press; 2002. Bones; pp. 1–380.
-
- Lanyon LE, Baggott DG. Mechanical function as an influence on the structure and form of bone. J Bone Joint Surg Br. 1976;58:436–43. - PubMed
-
- Turner CH. Bone strength: current concepts. Ann NY Acad Sci. 2006;1068:429–46. - PubMed
-
- Burr DB, Turner CH, Naick P, et al. Does microdamage accumulation affect the mechanical properties of bone? J Biomech. 1998;31:337–45. - PubMed
-
- Currey JD. Mechanical consequences of variation in the mineral content of bone. J Biomech. 1969;2:1–11. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

