The major chemical-detoxifying system of UDP-glucuronosyltransferases requires regulated phosphorylation supported by protein kinase C
- PMID: 18556656
- PMCID: PMC2516997
- DOI: 10.1074/jbc.M800032200
The major chemical-detoxifying system of UDP-glucuronosyltransferases requires regulated phosphorylation supported by protein kinase C
Abstract
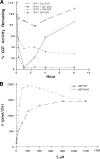
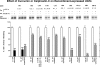
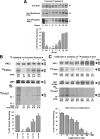
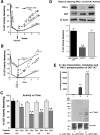
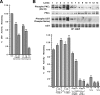
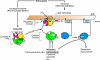
Finding rapid, reversible down-regulation of human UDP-glucuronosyltransferases (UGTs) in LS180 cells following curcumin treatment led to the discovery that UGTs require phosphorylation. UGTs, distributed primarily in liver, kidney, and gastrointestinal tract, inactivate aromatic-like metabolites and a vast number of dietary and environmental chemicals, which reduces the risk of toxicities, mutagenesis, and carcinogenesis. Our aim here is to determine relevant kinases and mechanism(s) regulating phosphorylation of constitutive UGTs in LS180 cells and 10 different human UGT cDNA-transfected COS-1 systems. Time- and concentration-dependent inhibition of immunodetectable [(33)P]orthophosphate in UGTs and protein kinase Cepsilon (PKCepsilon), following treatment of LS180 cells with curcumin or the PKC inhibitor calphostin-C, suggested UGT phosphorylation is supported by active PKC(s). Immunofluorescent and co-immunoprecipitation studies with UGT-transfected cells showed co-localization of UGT1A7His and PKCepsilon and of UGT1A10His and PKCalpha or PKCdelta. Inhibition of UGT activity by PKCepsilon-specific antagonist peptide or by PKCepsilon-targeted destruction with PKCepsilon-specific small interference RNA and activation of curcumin-down-regulated UGTs with typical PKC agonists verified a central PKC role in glucuronidation. Moreover, in vitro phosphorylation of nascent UGT1A7His by PKCepsilon confirms it is a bona fide PKC substrate. Finally, catalase or herbimycin-A inhibition of constitutive or hydrogen peroxide-activated-UGTs demonstrated that reactive oxygen species-related oxidants act as second messengers in maintaining constitutive PKC-dependent signaling evidently sustaining UGT phosphorylation and activity. Because cells use signal transduction collectively to detect and respond appropriately to environmental changes, this report, combined with our earlier demonstration that specific phospho-groups in UGT1A7 determined substrate selections, suggests regulated phosphorylation allows adaptations regarding differential phosphate utilization by UGTs to function efficiently.
Figures

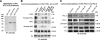
Similar articles
-
Phosphorylation of a UDP-glucuronosyltransferase regulates substrate specificity.Proc Natl Acad Sci U S A. 2005 May 3;102(18):6285-90. doi: 10.1073/pnas.0407872102. Epub 2005 Apr 21. Proc Natl Acad Sci U S A. 2005. PMID: 15845768 Free PMC article.
-
Protein kinase Cα and Src kinase support human prostate-distributed dihydrotestosterone-metabolizing UDP-glucuronosyltransferase 2B15 activity.J Biol Chem. 2012 Jul 13;287(29):24387-96. doi: 10.1074/jbc.M111.335067. Epub 2012 Apr 24. J Biol Chem. 2012. PMID: 22532564 Free PMC article.
-
Interpretation of the effects of protein kinase C inhibitors on human UDP-glucuronosyltransferase 1A (UGT1A) proteins in cellulo.Drug Metab Pharmacokinet. 2011 Jun;26(3):256-65. doi: 10.2133/dmpk.DMPK-10-RG-121. Epub 2011 Feb 8. Drug Metab Pharmacokinet. 2011. PMID: 21317540
-
Transcriptional regulation of human UDP-glucuronosyltransferase genes.Drug Metab Rev. 2014 Nov;46(4):421-58. doi: 10.3109/03602532.2014.973037. Epub 2014 Oct 22. Drug Metab Rev. 2014. PMID: 25336387 Review.
-
A historical overview of the heterologous expression of mammalian UDP-glucuronosyltransferase isoforms over the past twenty years.Curr Drug Metab. 2005 Apr;6(2):141-60. doi: 10.2174/1389200053586127. Curr Drug Metab. 2005. PMID: 15853765 Review.
Cited by
-
Role for protein kinase C delta in the functional activity of human UGT1A6: implications for drug-drug interactions between PKC inhibitors and UGT1A6.Xenobiotica. 2010 May;40(5):306-18. doi: 10.3109/00498251003596817. Xenobiotica. 2010. PMID: 20196639 Free PMC article.
-
The Inhibitory Activity of Curcumin on P-Glycoprotein and Its Uptake by and Efflux from LS180 Cells Is Not Affected by Its Galenic Formulation.Antioxidants (Basel). 2021 Nov 17;10(11):1826. doi: 10.3390/antiox10111826. Antioxidants (Basel). 2021. PMID: 34829695 Free PMC article.
-
Homology Modeling of Human Uridine-5'-diphosphate-glucuronosyltransferase 1A6 Reveals Insights into Factors Influencing Substrate and Cosubstrate Binding.ACS Omega. 2020 Mar 20;5(12):6872-6887. doi: 10.1021/acsomega.0c00205. eCollection 2020 Mar 31. ACS Omega. 2020. PMID: 32258923 Free PMC article.
-
Effect of a herbal extract containing curcumin and piperine on midazolam, flurbiprofen and paracetamol (acetaminophen) pharmacokinetics in healthy volunteers.Br J Clin Pharmacol. 2013 Feb;75(2):450-62. doi: 10.1111/j.1365-2125.2012.04364.x. Br J Clin Pharmacol. 2013. PMID: 22725836 Free PMC article. Clinical Trial.
-
First-pass metabolism via UDP-glucuronosyltransferase: a barrier to oral bioavailability of phenolics.J Pharm Sci. 2011 Sep;100(9):3655-81. doi: 10.1002/jps.22568. Epub 2011 Apr 11. J Pharm Sci. 2011. PMID: 21484808 Free PMC article. Review.
References
-
- Dutton, G. J. (1980) in Glucuronidation of Drugs and Other Compounds (Dutton, G. J., ed) pp. 69–78, CRC Press, Boca Raton, FL
-
- Ritter, J. K., Crawford, J. M., and Owens, I. S. (1991) J. Biol. Chem. 266 1043–1047 - PubMed
-
- Wells, P. G., Mackenzie, P. I., Chowdhury, J. R., Guillemette, C., Gregory, P. A., Ishii, Y., Hansen, A. J., Kessler, F. K., Kim, P. M., Chowdhury, N. R., and Ritter, J. K. (2004) Drug Metab. Dispos. 32 281–290 - PubMed
-
- Basu, N. K., Ciotti, M., Hwang, M. S., Kole, L., Mitra, P. S., Cho, J. W., and Owens, I. S. (2004) J. Biol. Chem. 279 1429–1441 - PubMed
-
- Basu, N. K., Kole, L., and Owens, I. S. (2003) Biochem. Biophys. Res. Commun. 303 98–104 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

