Mutation of FIG4 causes a rapidly progressive, asymmetric neuronal degeneration
- PMID: 18556664
- PMCID: PMC2724900
- DOI: 10.1093/brain/awn114
Mutation of FIG4 causes a rapidly progressive, asymmetric neuronal degeneration
Abstract
Recessive Charcot-Marie-Tooth disease type-4J (CMT4J) and its animal model, the pale tremor mouse (plt), are caused by mutations of the FIG4 gene encoding a PI(3,5)P(2) 5-phosphatase. We describe the 9-year clinical course of CMT4J, including asymmetric, rapidly progressive paralysis, in two siblings. Sensory symptoms were absent despite reduced numbers of sensory axons. Thus, the phenotypic presentation of CMT4J clinically resembles motor neuron disease. Time-lapse imaging of fibroblasts from CMT4J patients demonstrates impaired trafficking of intracellular organelles because of obstruction by vacuoles. Further characterization of plt mice identified axonal degeneration in motor and sensory neurons, limited segmental demyelination, lack of TUNEL staining and lack of accumulation of ubiquitinated protein in vacuoles of motor and sensory neurons. This study represents the first documentation of the natural history of CMT4J. Physical obstruction of organelle trafficking by vacuoles is a potential novel cellular mechanism of neurodegeneration.
Figures


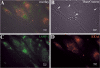

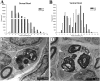


References
-
- Bai YH, Ianokova E, Pu Q, Ghandour K, Levinson R, Martin JJ, et al. R69C Mutation in P0 Gene alters myelination and ion channel subtypes. Arch Neurol. 2006;63:1787–94. - PubMed
-
- Ballar P, Shen Y, Yang H, Fang S. The role of a novel p97/valosin-containing protein-interacting motif of gp78 in endoplasmic reticulum-associated degradation. J Biol Chem. 2006;281:35359–68. - PubMed
-
- Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat Rev Neurosci. 2007;8:663–72. - PubMed
-
- Barber RP, Phelps PE, Houser CR, Crawford GD, Salvaterra PM, Vaughn JE. The morphology and distribution of neurons containing choline acetyltransferase in the adult rat spinal cord: an immunocytochemical study. J Comp Neurol. 1984;229:329–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

