Highly efficient circulating tumor cell isolation from whole blood and label-free enumeration using polymer-based microfluidics with an integrated conductivity sensor
- PMID: 18557614
- PMCID: PMC2526315
- DOI: 10.1021/ja8015022
Highly efficient circulating tumor cell isolation from whole blood and label-free enumeration using polymer-based microfluidics with an integrated conductivity sensor
Abstract
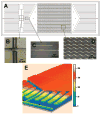
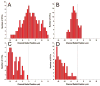
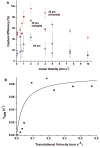
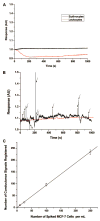
A novel microfluidic device that can selectively and specifically isolate exceedingly small numbers of circulating tumor cells (CTCs) through a monoclonal antibody (mAB) mediated process by sampling large input volumes (>/=1 mL) of whole blood directly in short time periods (<37 min) was demonstrated. The CTCs were concentrated into small volumes (190 nL), and the number of cells captured was read without labeling using an integrated conductivity sensor following release from the capture surface. The microfluidic device contained a series (51) of high-aspect ratio microchannels (35 mum width x 150 mum depth) that were replicated in poly(methyl methacrylate), PMMA, from a metal mold master. The microchannel walls were covalently decorated with mABs directed against breast cancer cells overexpressing the epithelial cell adhesion molecule (EpCAM). This microfluidic device could accept inputs of whole blood, and its CTC capture efficiency was made highly quantitative (>97%) by designing capture channels with the appropriate widths and heights. The isolated CTCs were readily released from the mAB capturing surface using trypsin. The released CTCs were then enumerated on-device using a novel, label-free solution conductivity route capable of detecting single tumor cells traveling through the detection electrodes. The conductivity readout provided near 100% detection efficiency and exquisite specificity for CTCs due to scaling factors and the nonoptimal electrical properties of potential interferences (erythrocytes or leukocytes). The simplicity in manufacturing the device and its ease of operation make it attractive for clinical applications requiring one-time use operation.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

