Chronic aspiration of gastric fluid induces the development of obliterative bronchiolitis in rat lung transplants
- PMID: 18557728
- PMCID: PMC5485647
- DOI: 10.1111/j.1600-6143.2008.02298.x
Chronic aspiration of gastric fluid induces the development of obliterative bronchiolitis in rat lung transplants
Abstract
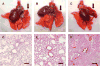
Long-term survival of a pulmonary allograft is currently hampered by obliterative bronchiolitis (OB), a form of chronic rejection that is unique to lung transplantation. While tracheobronchial aspiration from gastroesophageal reflux disease (GERD) has clinically been associated with OB, no experimental model exists to investigate this problem. Using a WKY-to-F344 rat orthotopic left lung transplant model, the effects of chronic aspiration on pulmonary allograft were evaluated. Recipients received cyclosporine with or without 8 weekly aspirations of gastric fluid into the allograft. Six (66.7%) of 9 allografts with aspiration demonstrated bronchioles with surrounding monocytic infiltrates, fibrosis and loss of normal lumen anatomy, consistent with the development of OB. In contrast, none of the allografts without aspiration (n = 10) demonstrated these findings (p = 0.002). Of the grafts examined grossly, 83% of the allografts with chronic aspiration but only 20% without aspiration appeared consolidated (p = 0.013). Aspiration was associated with increased levels of IL-1 alpha, IL-1 beta, IL-6, IL-10, TNF-alpha and TGF-beta in BAL and of IL-1 alpha, IL-4 and GM-CSF in serum. This study provides experimental evidence linking chronic aspiration to the development of OB and suggests that strategies aimed at preventing aspiration-related injuries might improve outcomes in clinical lung transplantation.
Figures


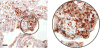
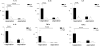
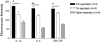
References
-
- Trulock EP, Edwards LB, Taylor DO, Boucek MM, Keck BM, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: Twenty-second official adult lung and heart-lung transplant report–2005. J Heart Lung Transplant. 2005;24:956–967. - PubMed
-
- Burke CM, Theodore J, Dawkins KD, et al. Post-transplant obliterative bronchiolitis and other late lung sequelae in human heart-lung transplantation. Chest. 1984;86:824–829. - PubMed
-
- Girgis RE, Tu I, Berry GJ, et al. Risk factors for the development of obliterative bronchiolitis after lung transplantation. J Heart Lung Transplant. 1996;15:1200–1208. - PubMed
-
- Fisher AJ, Wardle J, Dark JH, Corris PA. Non-immune acute graft injury after lung transplantation and the risk of subsequent bronchiolitis obliterans syndrome (BOS) J Heart Lung Transplant. 2002;21:1206–1212. - PubMed
-
- Reid KR, McKenzie FN, Menkis AH, et al. Importance of chronic aspiration in recipients of heart-lung transplants. Lancet. 1990;336:206–208. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

