Chemical and genetic validation of dihydrofolate reductase-thymidylate synthase as a drug target in African trypanosomes
- PMID: 18557814
- PMCID: PMC2610392
- DOI: 10.1111/j.1365-2958.2008.06305.x
Chemical and genetic validation of dihydrofolate reductase-thymidylate synthase as a drug target in African trypanosomes
Abstract
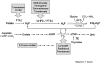
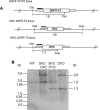
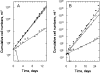
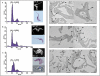
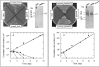
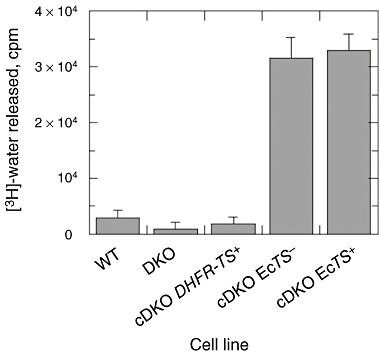
The phenotypes of single- (SKO) and double-knockout (DKO) lines of dihydrofolate reductase-thymidylate synthase (DHFR-TS) of bloodstream Trypanosoma brucei were evaluated in vitro and in vivo. Growth of SKO in vitro is identical to wild-type (WT) cells, whereas DKO has an absolute requirement for thymidine. Removal of thymidine from the medium triggers growth arrest in S phase, associated with gross morphological changes, followed by cell death after 60 h. DKO is unable to infect mice, whereas the virulence of SKO is similar to WT. Normal growth and virulence could be restored by transfection of DKO with T. brucei DHFR-TS, but not with Escherichia coli TS. As pteridine reductase (PTR1) levels are unchanged in SKO and DKO cells, PTR1 is not able to compensate for loss of DHFR activity. Drugs such as raltitrexed or methotrexate with structural similarity to folic acid are up to 300-fold more potent inhibitors of WT cultured in a novel low-folate medium, unlike hydrophobic antifols such as trimetrexate or pyrimethamine. DKO trypanosomes show reduced sensitivity to these inhibitors ranging from twofold for trimetrexate to >10 000-fold for raltitrexed. These data demonstrate that DHFR-TS is essential for parasite survival and represents a promising target for drug discovery.
Figures
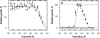


References
-
- Ackland SP, Beale P, Peters GJ. Thymidylate synthase inhibitors. Cancer Chemother Biol Response Modif. 2003;21:1–28. - PubMed
-
- Ahmad SI, Kirk SH, Eisenstark A. Thymine metabolism and thymineless death in prokaryotes and eukaryotes. Annu Rev Microbiol. 1998;52:591–625. - PubMed
-
- Backus HH, Pinedo HM, Wouters D, Padron JM, Molders N, Der Wilt CL, et al. Folate depletion increases sensitivity of solid tumor cell lines to 5-fluorouracil and antifolates. Int J Cancer. 2000;87:771–778. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

