Regulation of SRF/CArG-dependent gene transcription during chronic partial obstruction of murine small intestine
- PMID: 18557893
- PMCID: PMC8320440
- DOI: 10.1111/j.1365-2982.2008.01149.x
Regulation of SRF/CArG-dependent gene transcription during chronic partial obstruction of murine small intestine
Abstract
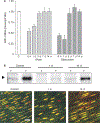
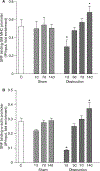
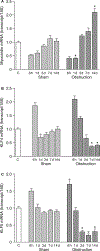
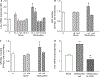
Intestinal obstructions lead to a variety of motility disorders. Small intestine smooth muscles undergo dramatic phenotypic changes in response to obstruction, but the underlying molecular mechanisms are unknown. Using RT-PCR, ChIP, Re-ChIP, and Western blots, we examined the effect of small bowel mechanical obstruction on smooth muscle gene expression. Obstruction caused a transient hyperplasia, followed by a prolonged hypertrophic response of small intestine smooth muscle cells. Smooth muscle myosin heavy chain (MHC), alpha-actin, and gamma-actin expression decreased initially, and then increased as hypertrophy developed. Myocardin expression decreased initially and then increased, while kruppel-like factors (KLF)4 and KLF5 expression increased initially, and then decreased. Serum response factor (SRF) expression decreased initially, and then recovered to sham-operated levels as hypertrophy developed. SRF binding to smooth muscle MHC and alpha-actin promoters decreased initially, but then increased above sham-operated levels as hypertrophy developed. Elk-1 binding to smooth muscle myosin heavy chain and alpha-actin promoters increased initially, and then decreased to sham-operated levels as hypertrophy developed. c-fos expression increased initially, which was associated with increased SRF/Elk-1 binding to the c-fos promoter. The Elk-1 phosphorylation inhibitor U-0126 inhibited the increase in c-fos expression. These findings indicate a dynamic response of small intestine smooth muscles to bowel obstruction involving switching between differentiated, proliferative, and hypertrophic phenotypes. These results suggest that changes in the expression and interactions between SRF, myocardin, Elk-1, and c-fos play key roles in the phenotypic switching of small intestine smooth muscles in response to mechanical obstruction.
Figures



References
-
- Chitkara DK, Di LC. From the bench to the ‘crib’-side: implications of scientific advances to paediatric neurogastroenterology and motility. Neurogastroenterol Motil 2006; 18: 251–62. - PubMed
-
- Jones MP, Wessinger S. Small intestinal motility. Curr Opin Gastroenterol 2006; 22: 111–6. - PubMed
-
- Gabella G Hypertrophy of visceral smooth muscle. Anat Embryol (Berl) 1990; 182: 409–24. - PubMed
-
- Storkholm JH, Zhao J, Villadsen GE, Hager H, Jensen SL, Gregersen H. Biomechanical remodeling of the chronically obstructed Guinea pig small intestine. Dig Dis Sci 2007; 52: 336–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

