Quantification of ligand-regulated nuclear receptor corepressor and coactivator binding, key interactions determining ligand potency and efficacy for the thyroid hormone receptor
- PMID: 18558711
- PMCID: PMC2574600
- DOI: 10.1021/bi800393u
Quantification of ligand-regulated nuclear receptor corepressor and coactivator binding, key interactions determining ligand potency and efficacy for the thyroid hormone receptor
Abstract
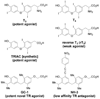
The potency and efficacy of ligands for nuclear receptors (NR) result both from the affinity of the ligand for the receptor and from the affinity that various coregulatory proteins have for ligand-receptor complexes; the latter interaction, however, is rarely quantified. To understand the molecular basis for ligand potency and efficacy, we developed dual time-resolved fluorescence resonance energy transfer (tr-FRET) assays and quantified binding of both ligand and coactivator or corepressor to the thyroid hormone receptor (TR). Promoter-bound TR exerts dual transcriptional regulatory functions, recruiting corepressor proteins and repressing transcription in the absence of thyroid hormones (THs) and shedding corepressors in favor of coactivators upon binding agonists, activating transcription. Our tr-FRET assays involve a TRE sequence labeled with terbium (fluorescence donor), TRbeta.RXRalpha heterodimer, and fluorescein-labeled NR interaction domains of coactivator SRC3 or corepressor NCoR (fluorescence acceptors). Through coregulator titrations, we could determine the affinity of SRC3 or NCoR for TRE-bound TR.RXR heterodimers, unliganded or saturated with different THs. Alternatively, through ligand titrations, we could determine the relative potencies of different THs. The order of TR agonist potencies is as follows: GC-1 approximately T 3 approximately TRIAC approximately T 4 >> rT 3 (for both coactivator recruitment and corepressor dissociation); the affinities of SRC3 binding to TR-ligand complexes followed a similar trend. This highlights the fact that the low activity of rT 3 is derived both from its low affinity for TR and from the low affinity of SRC for the TR-rT 3 complex. The TR antagonist NH-3 failed to induce SRC3 recruitment but did effect NCoR dissociation. These assays provide quantitative information about the affinity of two key interactions that are determinants of NR ligand potency and efficacy.
Figures
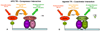


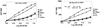




Similar articles
-
A dual-acceptor time-resolved Föster resonance energy transfer assay for simultaneous determination of thyroid hormone regulation of corepressor and coactivator binding to the thyroid hormone receptor: Mimicking the cellular context of thyroid hormone action.Anal Biochem. 2009 Mar 1;386(1):73-8. doi: 10.1016/j.ab.2008.11.039. Epub 2008 Dec 7. Anal Biochem. 2009. PMID: 19111515 Free PMC article.
-
Defective release of corepressor by hinge mutants of the thyroid hormone receptor found in patients with resistance to thyroid hormone.J Biol Chem. 1998 Nov 13;273(46):30175-82. doi: 10.1074/jbc.273.46.30175. J Biol Chem. 1998. PMID: 9804773
-
Pathological Interactions Between Mutant Thyroid Hormone Receptors and Corepressors and Their Modulation by a Thyroid Hormone Analogue with Therapeutic Potential.Thyroid. 2018 Dec;28(12):1708-1722. doi: 10.1089/thy.2017.0551. Epub 2018 Oct 16. Thyroid. 2018. PMID: 30235988
-
Molecular and structural biology of thyroid hormone receptors.Clin Exp Pharmacol Physiol Suppl. 1998 Nov;25:S2-11. doi: 10.1111/j.1440-1681.1998.tb02293.x. Clin Exp Pharmacol Physiol Suppl. 1998. PMID: 9809185 Review.
-
Role of co-activators and co-repressors in the mechanism of steroid/thyroid receptor action.Recent Prog Horm Res. 1997;52:141-64; discussion 164-5. Recent Prog Horm Res. 1997. PMID: 9238851 Review.
Cited by
-
Structure of a thyroid hormone receptor DNA-binding domain homodimer bound to an inverted palindrome DNA response element.Mol Endocrinol. 2010 Aug;24(8):1650-64. doi: 10.1210/me.2010-0129. Epub 2010 Jul 7. Mol Endocrinol. 2010. PMID: 20610536 Free PMC article.
-
Nuclear receptors and epigenetic regulation: opportunities for nutritional targeting and disease prevention.Adv Nutr. 2014 Jul 14;5(4):373-85. doi: 10.3945/an.114.005868. Print 2014 Jul. Adv Nutr. 2014. PMID: 25022987 Free PMC article. Review.
-
Assessing dose-response relationships for endocrine disrupting chemicals (EDCs): a focus on non-monotonicity.Environ Health. 2015 May 15;14:42. doi: 10.1186/s12940-015-0029-4. Environ Health. 2015. PMID: 25971795 Free PMC article.
-
Development of time resolved fluorescence resonance energy transfer-based assay for FXR antagonist discovery.Bioorg Med Chem. 2013 Jul 15;21(14):4266-78. doi: 10.1016/j.bmc.2013.04.069. Epub 2013 May 7. Bioorg Med Chem. 2013. PMID: 23688559 Free PMC article.
-
Non-monotonic dose responses in studies of endocrine disrupting chemicals: bisphenol a as a case study.Dose Response. 2013 Oct 7;12(2):259-76. doi: 10.2203/dose-response.13-020.Vandenberg. eCollection 2014 May. Dose Response. 2013. PMID: 24910584 Free PMC article.
References
-
- Lonard DM, O'Malley BW. Expanding functional diversity of the coactivators. Trends Biochem. Sci. 2005;30:126–132. - PubMed
-
- Privalsky ML. The role of corepressors in transcriptional regulation by nuclear hormone receptors. Annu. Rev. Physiol. 2004;66:315–360. - PubMed
-
- Yen PM, Ando S, Feng X, Liu Y, Maruvada P, Xia X. Thyroid hormone action at the cellular, genomic and target gene levels. Mol. Cell. Endocrinol. 2006;246:121–127. - PubMed
-
- Lazar MA. Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr. Rev. 1993;14:184–193. - PubMed
-
- Apriletti JW, Eberhardt NL, Latham KR, Baxter JD. Affinity chromatography of thyroid hormone receptors. Biospecific elution from support matrices, characterization of the partially purified receptor. J. Biol. Chem. 1981;256:12094–12101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

