Brain circuits for the internal monitoring of movements
- PMID: 18558858
- PMCID: PMC2813694
- DOI: 10.1146/annurev.neuro.31.060407.125627
Brain circuits for the internal monitoring of movements
Abstract
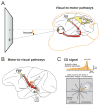
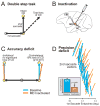
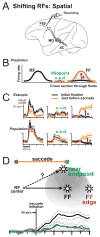
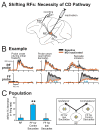
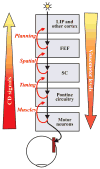
Each movement we make activates our own sensory receptors, thus causing a problem for the brain: the spurious, movement-related sensations must be discriminated from the sensory inputs that really matter, those representing our environment. Here we consider circuits for solving this problem in the primate brain. Such circuits convey a copy of each motor command, known as a corollary discharge (CD), to brain regions that use sensory input. In the visual system, CD signals may help to produce a stable visual percept from the jumpy images resulting from our rapid eye movements. A candidate pathway for providing CD for vision ascends from the superior colliculus to the frontal cortex in the primate brain. This circuit conveys warning signals about impending eye movements that are used for planning subsequent movements and analyzing the visual world. Identifying this circuit has provided a model for studying CD in other primate sensory systems and may lead to a better understanding of motor and mental disorders.
Figures




References
-
- Andersen RA, Buneo CA. Intentional maps in posterior parietal cortex. Annu Rev Neurosci. 2002;25:189–220. - PubMed
-
- Andreasen NC, Nopoulos P, O'Leary DS, Miller DD, Wassink T, Flaum M. Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biol Psychiatry. 1999;46:908–20. - PubMed
-
- Batista AP, Buneo CA, Snyder LH, Andersen RA. Reach plans in eye-centered coordinates. Science. 1999;285:257–60. - PubMed
-
- Bell CC. Properties of a modifiable efference copy in an electric fish. J Neurophysiol. 1982;47:1043–56. - PubMed
-
- Bellebaum C, Daum I, Koch B, Schwarz M, Hoffmann KP. The role of the human thalamus in processing corollary discharge. Brain. 2005;128:1139–54. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

