Changes in corticospinal excitability and the direction of evoked movements during motor preparation: a TMS study
- PMID: 18559096
- PMCID: PMC2453131
- DOI: 10.1186/1471-2202-9-51
Changes in corticospinal excitability and the direction of evoked movements during motor preparation: a TMS study
Abstract
Background: Preparation of the direction of a forthcoming movement has a particularly strong influence on both reaction times and neuronal activity in the primate motor cortex. Here, we aimed to find direct neurophysiologic evidence for the preparation of movement direction in humans. We used single-pulse transcranial magnetic stimulation (TMS) to evoke isolated thumb-movements, of which the direction can be modulated experimentally, for example by training or by motor tasks. Sixteen healthy subjects performed brisk concentric voluntary thumb movements during a reaction time task in which the required movement direction was precued. We assessed whether preparation for the thumb movement lead to changes in the direction of TMS-evoked movements and to changes in amplitudes of motor-evoked potentials (MEPs) from the hand muscles.
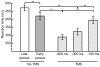
Results: When the required movement direction was precued early in the preparatory interval, reaction times were 50 ms faster than when precued at the end of the preparatory interval. Over time, the direction of the TMS-evoked thumb movements became increasingly variable, but it did not turn towards the precued direction. MEPs from the thumb muscle (agonist) were differentially modulated by the direction of the precue, but only in the late phase of the preparatory interval and thereafter. MEPs from the index finger muscle did not depend on the precued direction and progressively decreased during the preparatory interval.
Conclusion: Our data show that the human corticospinal movement representation undergoes progressive changes during motor preparation. These changes are accompanied by inhibitory changes in corticospinal excitability, which are muscle specific and depend on the prepared movement direction. This inhibition might indicate a corticospinal braking mechanism that counteracts any preparatory motor activation.
Figures


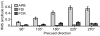





Similar articles
-
Reversal of TMS-induced motor twitch by training is associated with a reduction in excitability of the antagonist muscle.J Neuroeng Rehabil. 2011 Aug 24;8:46. doi: 10.1186/1743-0003-8-46. J Neuroeng Rehabil. 2011. PMID: 21861922 Free PMC article.
-
Concurrent action observation modulates practice-induced motor memory formation.Eur J Neurosci. 2008 Feb;27(3):730-8. doi: 10.1111/j.1460-9568.2008.06035.x. Eur J Neurosci. 2008. PMID: 18279325
-
Reversal of Practice-related Effects on Corticospinal Excitability has no Immediate Effect on Behavioral Outcome.Brain Stimul. 2015 May-Jun;8(3):603-12. doi: 10.1016/j.brs.2015.01.405. Epub 2015 Jan 21. Brain Stimul. 2015. PMID: 25697591
-
Moving forward: methodological considerations for assessing corticospinal excitability during rhythmic motor output in humans.J Neurophysiol. 2021 Jul 1;126(1):181-194. doi: 10.1152/jn.00027.2021. Epub 2021 Jun 16. J Neurophysiol. 2021. PMID: 34133230 Review.
-
Motor potentials evoked by transcranial magnetic stimulation: interpreting a simple measure of a complex system.J Physiol. 2023 Jul;601(14):2827-2851. doi: 10.1113/JP281885. Epub 2023 Jun 8. J Physiol. 2023. PMID: 37254441 Free PMC article. Review.
Cited by
-
Corticospinal Modulations during Bimanual Movement with Different Relative Phases.Front Hum Neurosci. 2016 Mar 7;10:95. doi: 10.3389/fnhum.2016.00095. eCollection 2016. Front Hum Neurosci. 2016. PMID: 27014026 Free PMC article.
-
Long-term practice of isolated finger movements reduces enslaved response of tonically contracting little finger abductor to tonic index finger abduction.Exp Brain Res. 2020 Feb;238(2):499-512. doi: 10.1007/s00221-020-05731-z. Epub 2020 Jan 20. Exp Brain Res. 2020. PMID: 31960102
-
Delay activity in rodent frontal cortex during a simple reaction time task.J Neurophysiol. 2009 Jun;101(6):2859-71. doi: 10.1152/jn.90615.2008. Epub 2009 Apr 1. J Neurophysiol. 2009. PMID: 19339463 Free PMC article.
-
Preparing to act follows Bayesian inference rules.iScience. 2025 May 12;28(6):112645. doi: 10.1016/j.isci.2025.112645. eCollection 2025 Jun 20. iScience. 2025. PMID: 40510122 Free PMC article.
-
How thoughts give rise to action - conscious motor intention increases the excitability of target-specific motor circuits.PLoS One. 2013 Dec 26;8(12):e83845. doi: 10.1371/journal.pone.0083845. eCollection 2013. PLoS One. 2013. PMID: 24386291 Free PMC article.
References
-
- Anson JG, Hyland BI, Kotter R, Wickens JR. Parameter precuing and motor preparation. Motor Control. 2000;4:221–231. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

