The homotetrameric phosphoseryl-tRNA synthetase from Methanosarcina mazei exhibits half-of-the-sites activity
- PMID: 18559342
- PMCID: PMC2494909
- DOI: 10.1074/jbc.M801838200
The homotetrameric phosphoseryl-tRNA synthetase from Methanosarcina mazei exhibits half-of-the-sites activity
Abstract
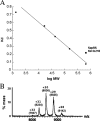
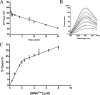
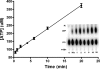
Synthesis of cysteinyl-tRNA(Cys) in methanogenic archaea proceeds by a two-step pathway in which tRNA(Cys) is first aminoacylated with phosphoserine by phosphoseryl-tRNA synthetase (SepRS). Characterization of SepRS from the mesophile Methanosarcina mazei by gel filtration and nondenaturing mass spectrometry shows that the native enzyme exists as an alpha4 tetramer when expressed at high levels in Escherichia coli. However, active site titrations monitored by ATP/PP(i) burst kinetics, together with analysis of tRNA binding stoichiometry by fluorescence spectroscopy, show that the tetrameric enzyme binds two tRNAs and that only two of the four chemically equivalent subunits catalyze formation of phosphoseryl adenylate. Therefore, the phenomenon of half-of-the-sites activity, previously described for synthesis of 1 mol of tyrosyl adenylate by the dimeric class I tyrosyl-tRNA synthetase, operates as well in this homotetrameric class II tRNA synthetase. Analysis of cognate and noncognate reactions by ATP/PP(i) and aminoacylation kinetics strongly suggests that SepRS is able to discriminate against the noncognate amino acids glutamate, serine, and phosphothreonine without the need for a separate hydrolytic editing site. tRNA(Cys) binding to SepRS also enhances the capacity of the enzyme to discriminate among amino acids, indicating the existence of functional connectivity between the tRNA and amino acid binding sites of the enzyme.
Figures

References
-
- Ibba, M., and Söll, D. (2004) Genes Dev. 18 731–738 - PubMed
-
- Sauerwald, A., Zhu, W., Major, T., Roy, H., Palioura, S., Jahn, D., Whitman, W. B., Yates, J. R., III, Ibba, M., and Söll, D. (2005) Science 307 1969–1972 - PubMed
-
- Tumbula, D. L., Becker, H. D., Chang, W. Z., and Söll, D. (2000) Nature 407 106–110 - PubMed
-
- Hanke, T., Bartmann, P., Hennecke, H., Kosakowski, H. M., Jaenicke, R., Holler, E., and Bock, A. (1974) Eur. J. Biochem. 43 601–607 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

