MPYS, a novel membrane tetraspanner, is associated with major histocompatibility complex class II and mediates transduction of apoptotic signals
- PMID: 18559423
- PMCID: PMC2519703
- DOI: 10.1128/MCB.00640-08
MPYS, a novel membrane tetraspanner, is associated with major histocompatibility complex class II and mediates transduction of apoptotic signals
Abstract
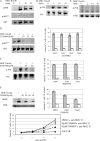
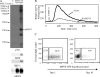
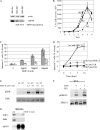
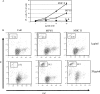
Although the best-defined function of type II major histocompatibility complex (MHC-II) is presentation of antigenic peptides to T lymphocytes, these molecules can also transduce signals leading alternatively to cell activation or apoptotic death. MHC-II is a heterodimer of two transmembrane proteins, each containing a short cytoplasmic tail that is dispensable for transduction of death signals. This suggests the function of an undefined MHC-II-associated transducer in signaling the death response. Here we describe a novel plasma membrane tetraspanner (MPYS) that is associated with MHC-II and mediates its transduction of death signals. MPYS is unusual among tetraspanners in containing an extended C-terminal cytoplasmic tail (approximately 140 amino acids) with multiple embedded signaling motifs. MPYS is tyrosine phosphorylated upon MHC-II aggregation and associates with inositol lipid and tyrosine phosphatases. Finally, MHC class II-mediated cell death signaling requires MPYS-dependent activation of the extracellular signal-regulated kinase signaling pathway.
Figures




References
-
- Al-Daccak, R., N. Mooney, and D. Charron. 2004. MHC class II signaling in antigen-presenting cells. Curr. Opin. Immunol. 16108-113. - PubMed
-
- Bertho, N., V. M. Blancheteau, N. Setterblad, B. Laupeze, J. M. Lord, B. Drenou, L. Amiot, D. J. Charron, R. Fauchet, and N. Mooney. 2002. MHC class II-mediated apoptosis of mature dendritic cells proceeds by activation of the protein kinase C-delta isoenzyme. Int. Immunol. 14935-942. - PubMed
-
- Bobbitt, K. R., and L. B. Justement. 2000. Regulation of MHC class II signal transduction by the B cell coreceptors CD19 and CD22. J. Immunol. 1655588-5596. - PubMed
-
- Bridges, S. H., A. M. Kruisbeek, and D. L. Longo. 1987. Selective in vivo antitumor effects of monoclonal anti-I-A antibody on B cell lymphoma. J. Immunol. 1394242-4249. - PubMed
-
- Brown, K. S., D. J. Levitt, M. Shannon, and B. K. Link. 2001. Phase II trial of Remitogen (humanized 1D10) monoclonal antibody targeting class II in patients with relapsed low-grade or follicular lymphoma. Clin. Lymphoma 2188-190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
