Escherichia coli DraE adhesin-associated bacterial internalization by epithelial cells is promoted independently by decay-accelerating factor and carcinoembryonic antigen-related cell adhesion molecule binding and does not require the DraD invasin
- PMID: 18559426
- PMCID: PMC2519432
- DOI: 10.1128/IAI.00427-08
Escherichia coli DraE adhesin-associated bacterial internalization by epithelial cells is promoted independently by decay-accelerating factor and carcinoembryonic antigen-related cell adhesion molecule binding and does not require the DraD invasin
Abstract
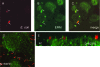
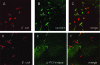
The Dr family of Escherichia coli adhesins are virulence factors associated with diarrhea and urinary tract infections. Dr fimbriae are comprised of two subunits. DraE/AfaE represents the major structural, antigenic, and adhesive subunit, which recognizes decay-accelerating factor (DAF) and carcinoembryonic antigen (CEA)-related cell adhesion molecules (CEACAMs) CEA, CEACAM1, CEACAM3, and CEACAM6 as binding receptors. The DraD/AfaD subunit caps fimbriae and has been implicated in the entry of Dr-fimbriated E. coli into host cells. In this study, we demonstrate that DAF or CEACAM receptors independently promote DraE-mediated internalization of E. coli by CHO cell transfectants expressing these receptors. We also found that DraE-positive recombinant bacteria adhere to and are internalized by primary human bladder epithelial cells which express DAF and CEACAMs. DraE-mediated bacterial internalization by bladder cells was inhibited by agents which disrupt lipid rafts, microtubules, and phosphatidylinositol 3-kinase (PI3K) activity. Immunofluorescence confocal microscopic examination of epithelial cells detected considerable recruitment of caveolin, beta(1) integrin, phosphorylated ezrin, phosphorylated PI3K, and tubulin, but not F-actin, by cell-associated bacteria. Finally, we demonstrate that the DraD subunit, previously implicated as an "invasin," is not required for beta(1) integrin recruitment or bacterial internalization.
Figures






Similar articles
-
Human decay-accelerating factor and CEACAM receptor-mediated internalization and intracellular lifestyle of Afa/Dr diffusely adhering Escherichia coli in epithelial cells.Infect Immun. 2009 Jan;77(1):517-31. doi: 10.1128/IAI.00695-08. Epub 2008 Nov 17. Infect Immun. 2009. PMID: 19015254 Free PMC article.
-
A subfamily of Dr adhesins of Escherichia coli bind independently to decay-accelerating factor and the N-domain of carcinoembryonic antigen.J Biol Chem. 2006 Sep 29;281(39):29120-30. doi: 10.1074/jbc.M605681200. Epub 2006 Aug 1. J Biol Chem. 2006. PMID: 16882658 Free PMC article.
-
Differential recognition of members of the carcinoembryonic antigen family by Afa/Dr adhesins of diffusely adhering Escherichia coli (Afa/Dr DAEC).Mol Microbiol. 2004 May;52(4):963-83. doi: 10.1111/j.1365-2958.2004.04033.x. Mol Microbiol. 2004. PMID: 15130118
-
Pathogenesis of Afa/Dr diffusely adhering Escherichia coli.Clin Microbiol Rev. 2005 Apr;18(2):264-92. doi: 10.1128/CMR.18.2.264-292.2005. Clin Microbiol Rev. 2005. PMID: 15831825 Free PMC article. Review.
-
Adherence of diarrheagenic Escherichia coli strains to epithelial cells.Infect Immun. 2005 Jan;73(1):18-29. doi: 10.1128/IAI.73.1.18-29.2005. Infect Immun. 2005. PMID: 15618137 Free PMC article. Review. No abstract available.
Cited by
-
Role of Src kinases in mobilization of glycosylphosphatidylinositol-anchored decay-accelerating factor by Dr fimbria-positive adhering bacteria.Infect Immun. 2011 Jul;79(7):2519-34. doi: 10.1128/IAI.01052-10. Epub 2011 Apr 25. Infect Immun. 2011. PMID: 21518786 Free PMC article.
-
Animal Models and Pathogenesis of Ulcerative Colitis.Comput Math Methods Med. 2022 Jul 11;2022:5927384. doi: 10.1155/2022/5927384. eCollection 2022. Comput Math Methods Med. 2022. Retraction in: Comput Math Methods Med. 2023 Jun 28;2023:9878915. doi: 10.1155/2023/9878915. PMID: 35860188 Free PMC article. Retracted.
-
A shear stress micromodel of urinary tract infection by the Escherichia coli producing Dr adhesin.PLoS Pathog. 2020 Jan 9;16(1):e1008247. doi: 10.1371/journal.ppat.1008247. eCollection 2020 Jan. PLoS Pathog. 2020. PMID: 31917805 Free PMC article.
-
Classical chaperone-usher (CU) adhesive fimbriome: uropathogenic Escherichia coli (UPEC) and urinary tract infections (UTIs).Folia Microbiol (Praha). 2020 Feb;65(1):45-65. doi: 10.1007/s12223-019-00719-x. Epub 2019 Jun 5. Folia Microbiol (Praha). 2020. PMID: 31165977 Review.
-
Virulence factors of uropathogens and their role in host pathogen interactions.Cell Surf. 2022 Feb 9;8:100075. doi: 10.1016/j.tcsw.2022.100075. eCollection 2022 Dec. Cell Surf. 2022. PMID: 35198842 Free PMC article.
References
-
- Abraham, S. N., M. J. Duncan, G. Li, and D. Zaas. 2005. Bacterial penetration of the mucosal barrier by targeting lipid rafts. J. Investig. Med. 53318-321. - PubMed
-
- Anderson, K. L., J. Billington, D. Pettigrew, E. Cota, P. Simpson, P. Roversi, H. A. Chen, P. Urvil, L. du Merle, P. N. Barlow, M. E. Medof, R. A. Smith, B. Nowicki, C. Le Bouguenec, S. M. Lea, and S. Matthews. 2004. An atomic resolution model for assembly, architecture, and function of the Dr adhesins. Mol. Cell 15647-657. - PubMed
-
- Berger, C. N., O. Billker, T. F. Meyer, A. L. Servin, and I. Kansau. 2004. Differential recognition of members of the carcinoembryonic antigen family by Afa/Dr adhesins of diffusely adhering Escherichia coli (Afa/Dr DAEC). Mol. Microbiol. 52963-983. - PubMed
-
- Blomfield, I. C., M. S. McClain, and B. I. Eisenstein. 1991. Type 1 fimbriae mutants of Escherichia coli K12: characterization of recognized afimbriate strains and construction of new fim deletion mutants. Mol. Microbiol. 51439-1445. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

