Identification of fevR, a novel regulator of virulence gene expression in Francisella novicida
- PMID: 18559431
- PMCID: PMC2493208
- DOI: 10.1128/IAI.00430-08
Identification of fevR, a novel regulator of virulence gene expression in Francisella novicida
Abstract
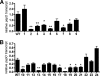
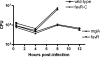
Francisella tularensis infects wild animals and humans to cause tularemia. This pathogen targets the cytosol of macrophages, where it replicates using the genes in the Francisella pathogenicity island (FPI). Virulence gene regulation in Francisella is complex, but transcriptional regulators MglA and SspA have been shown to regulate the expression of approximately 100 genes, including the entire FPI. We utilized a Francisella novicida transposon mutant library to identify additional regulatory factors and identified five additional genes that are essential for virulence gene expression. One regulatory gene, FTN_0480 (fevR, Francisella effector of virulence regulation), present in all Francisella species, is required for expression of the FPI genes and other genes in the MglA/SspA regulon. The expression of fevR is positively regulated by MglA. However, constitutive expression of fevR in an mglA mutant strain did not restore expression of the MglA/SspA regulon, demonstrating that mglA and fevR act in parallel to positively regulate virulence gene expression. Virulence studies revealed that fevR is essential for bacterial replication in macrophages and in mice, where we additionally show that fevR is required for the expression of genes in the MglA/SspA regulon in vivo. Thus, fevR is a crucial virulence gene in Francisella, required for the expression of virulence factors known to be essential for this pathogen's subversion of host defenses and pathogenesis in vivo.
Figures


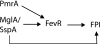
References
-
- Anthony, L. S., M. Z. Gu, S. C. Cowley, W. W. Leung, and F. E. Nano. 1991. Transformation and allelic replacement in Francisella spp. J. Gen. Microbiol. 1372697-2703. - PubMed
-
- Baron, G. S., and F. E. Nano. 1999. An erythromycin resistance cassette and mini-transposon for constructing transcriptional fusions to cat. Gene 22959-65. - PubMed
-
- Baron, G. S., and F. E. Nano. 1998. MglA and MglB are required for the intramacrophage growth of Francisella novicida. Mol. Microbiol. 29247-259. - PubMed
-
- Bosio, C. M., and S. W. Dow. 2005. Francisella tularensis induces aberrant activation of pulmonary dendritic cells. J. Immunol. 1756792-6801. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

