Molecular basis of uropathogenic Escherichia coli evasion of the innate immune response in the bladder
- PMID: 18559433
- PMCID: PMC2519411
- DOI: 10.1128/IAI.00069-08
Molecular basis of uropathogenic Escherichia coli evasion of the innate immune response in the bladder
Abstract
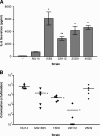
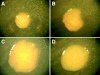
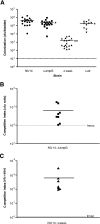
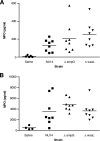
In the urinary tract, the innate immune system detects conserved bacterial components and responds to infection by activating the proinflammatory transcription factor NF-kappaB, resulting in cytokine secretion and neutrophil recruitment. Uropathogenic Escherichia coli (UPEC), however, has been shown to evade the host innate immune response by suppressing NF-kappaB activation in urothelial cells, which results in decreased cytokine secretion and increased urothelial apoptosis. To understand the molecular basis of UPEC modulation of inflammation, we performed a genetic screen with UPEC strain NU14 to identify genes which are required for modulation of urothelial cytokine secretion. Disruption of ampG (peptidoglycan permease), waaL (lipopolysaccharide O antigen ligase), or alr (alanine racemase) resulted in increased urothelial interleukin-8 (IL-8) and IL-6 release from urothelial cell cultures. Targeted deletion of these genes also resulted in elevated urothelial cytokine production during UPEC infection. Conditioned media from bacterial cultures of NU14 DeltaampG and NU14 DeltawaaL contained a heat-stable factor(s) which stimulated greater urothelial IL-8 secretion than that in NU14-conditioned medium. In a mouse model of urinary tract infection, NU14 DeltaampG, NU14 DeltawaaL, and NU14 Deltaalr were attenuated compared to wild-type NU14 and showed reduced fitness in competition experiments. Instillation of NU14 DeltaampG or NU14 DeltawaaL increased bladder neutrophil recruitment, indicating that enhanced urothelial cytokine secretion during urinary tract infection results in an altered host response. Thus, UPEC evasion of innate immune detection of bacterial components, such as lipopolysaccharide and peptidoglycan fragments, is likely an important factor in the ability of UPEC to colonize the urinary tract.
Figures



References
-
- Alexeyev, M. F., and I. N. Shokolenko. 1995. Mini-Tn10 transposon derivatives for insertion mutagenesis and gene delivery into the chromosome of gram-negative bacteria. Gene 16059-62. - PubMed
-
- Andersen-Nissen, E., T. R. Hawn, K. D. Smith, A. Nachman, A. E. Lampano, S. Uematsu, S. Akira, and A. Aderem. 2007. Cutting edge: Tlr5−/− mice are more susceptible to Escherichia coli urinary tract infection. J. Immunol. 1784717-4720. - PubMed
-
- Backhed, F., L. Meijer, S. Normark, and A. Richter-Dahlfors. 2002. TLR4-dependent recognition of lipopolysaccharide by epithelial cells requires sCD14. Cell. Microbiol. 4493-501. - PubMed
-
- Backhed, F., S. Normark, and A. Richter-Dahlfors. 2002. TLR4-dependent lipopolysaccharide signalling in epithelial cells is independent of extracellular protease activity. Cell. Microbiol. 4297-303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

