The anaphase-promoting complex/cyclosome (APC/C) is required for rereplication control in endoreplication cycles
- PMID: 18559483
- PMCID: PMC2428065
- DOI: 10.1101/gad.469108
The anaphase-promoting complex/cyclosome (APC/C) is required for rereplication control in endoreplication cycles
Abstract
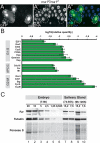
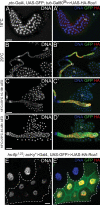
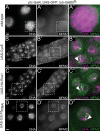
Endoreplicating cells undergo multiple rounds of DNA replication leading to polyploidy or polyteny. Oscillation of Cyclin E (CycE)-dependent kinase activity is the main driving force in Drosophila endocycles. High levels of CycE-Cdk2 activity trigger S phase, while down-regulation of CycE-Cdk2 activity is crucial to allow licensing of replication origins. In mitotic cells relicensing in S phase is prevented by Geminin. Here we show that Geminin protein oscillates in endoreplicating salivary glands of Drosophila. Geminin levels are high in S phase, but drop once DNA replication has been completed. DNA licensing is coupled to mitosis through the action of the anaphase-promoting complex/cyclosome (APC/C). We demonstrate that, even though endoreplicating cells never enter mitosis, APC/C activity is required in endoreplicating cells to mediate Geminin oscillation. Down-regulation of APC/C activity results in stabilization of Geminin protein and blocks endocycle progression. Geminin is only abundant in cells with high CycE-Cdk2 activity, suggesting that APC/C-Fzr activity is periodically inhibited by CycE-Cdk2, to prevent relicensing in S-phase cells.
Figures




Similar articles
-
The spindle checkpoint, APC/C(Cdc20), and APC/C(Cdh1) play distinct roles in connecting mitosis to S phase.J Cell Biol. 2013 Jun 24;201(7):1013-26. doi: 10.1083/jcb.201211019. Epub 2013 Jun 17. J Cell Biol. 2013. PMID: 23775192 Free PMC article.
-
APC/CFzr/Cdh1 promotes cell cycle progression during the Drosophila endocycle.Development. 2008 Apr;135(8):1451-61. doi: 10.1242/dev.016295. Epub 2008 Mar 5. Development. 2008. PMID: 18321983
-
Regulation of early events in chromosome replication.Curr Biol. 2004 Sep 21;14(18):R778-86. doi: 10.1016/j.cub.2004.09.019. Curr Biol. 2004. PMID: 15380092 Review.
-
Nonperiodic activity of the human anaphase-promoting complex-Cdh1 ubiquitin ligase results in continuous DNA synthesis uncoupled from mitosis.Mol Cell Biol. 2000 Oct;20(20):7613-23. doi: 10.1128/MCB.20.20.7613-7623.2000. Mol Cell Biol. 2000. PMID: 11003657 Free PMC article.
-
Cdt1 interactions in the licensing process: a model for dynamic spatiotemporal control of licensing.Cell Cycle. 2007 Jul 1;6(13):1549-52. doi: 10.4161/cc.6.13.4455. Epub 2007 May 22. Cell Cycle. 2007. PMID: 17598984 Review.
Cited by
-
Cip/Kip cyclin-dependent protein kinase inhibitors and the road to polyploidy.Cell Div. 2009 Jun 2;4:10. doi: 10.1186/1747-1028-4-10. Cell Div. 2009. PMID: 19490616 Free PMC article.
-
ABL1-mediated phosphorylation promotes FOXM1-related tumorigenicity by Increasing FOXM1 stability.Cell Death Differ. 2024 Oct;31(10):1285-1301. doi: 10.1038/s41418-024-01339-w. Epub 2024 Jul 26. Cell Death Differ. 2024. PMID: 39060421 Free PMC article.
-
APC/CCdh1 Enables Removal of Shugoshin-2 from the Arms of Bivalent Chromosomes by Moderating Cyclin-Dependent Kinase Activity.Curr Biol. 2017 May 22;27(10):1462-1476.e5. doi: 10.1016/j.cub.2017.04.023. Epub 2017 May 11. Curr Biol. 2017. PMID: 28502659 Free PMC article.
-
The spindle checkpoint, APC/C(Cdc20), and APC/C(Cdh1) play distinct roles in connecting mitosis to S phase.J Cell Biol. 2013 Jun 24;201(7):1013-26. doi: 10.1083/jcb.201211019. Epub 2013 Jun 17. J Cell Biol. 2013. PMID: 23775192 Free PMC article.
-
The microRNA bantam regulates a developmental transition in epithelial cells that restricts sensory dendrite growth.Development. 2014 Jul;141(13):2657-68. doi: 10.1242/dev.107573. Epub 2014 Jun 12. Development. 2014. PMID: 24924190 Free PMC article.
References
-
- Arias E.E., Walter J.C. Strength in numbers: Preventing rereplication via multiple mechanisms in eukaryotic cells. Genes & Dev. 2007;21:497–518. - PubMed
-
- Asano M., Nevins J.R., Wharton R.P. Ectopic E2F expression induces S phase and apoptosis in Drosophila imaginal discs. Genes & Dev. 1996;10:1422–1432. - PubMed
-
- Baker N.E., Yu S.Y. The EGF receptor defines domains of cell cycle progression and survival to regulate cell number in the developing Drosophila eye. Cell. 2001;104:699–708. - PubMed
-
- Bell S.P., Dutta A. DNA replication in eukaryotic cells. Annu. Rev. Biochem. 2002;71:333–374. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
