Elevated CRAF as a potential mechanism of acquired resistance to BRAF inhibition in melanoma
- PMID: 18559533
- PMCID: PMC2692356
- DOI: 10.1158/0008-5472.CAN-07-6787
Elevated CRAF as a potential mechanism of acquired resistance to BRAF inhibition in melanoma
Abstract
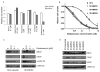
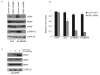
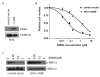
Activating BRAF kinase mutations arise in approximately 7% of all human tumors, and preclinical studies have validated the RAF-mitogen-activated protein/extracellular signal-regulated kinase (ERK) kinase-ERK signaling cascade as a potentially important therapeutic target in this setting. Selective RAF kinase inhibitors are currently undergoing clinical development, and based on the experience with other kinase-targeted therapeutics, it is expected that clinical responses to these agents, if observed, will lead to the eventual emergence of drug resistance in most cases. Thus, it is important to establish molecular mechanisms underlying such resistance to develop effective therapeutic strategies to overcome or prevent drug resistance. To anticipate potential mechanisms of acquired resistance to RAF inhibitors during the course of treatment, we established drug-resistant clones from a human melanoma-derived cell line harboring the recurrent V600E activating BRAF mutation, which exhibits exquisite sensitivity to AZ628, a selective RAF kinase inhibitor. We determined that elevated CRAF protein levels account for the acquisition of resistance to AZ628 in these cells, associated with a switch from BRAF to CRAF dependency in tumor cells. We also found that elevated CRAF protein levels may similarly contribute to primary insensitivity to RAF inhibition in a subset of BRAF mutant tumor cells. Interestingly, AZ628-resistant cells demonstrating either primary drug insensitivity or acquired drug resistance exhibit exquisite sensitivity to the HSP90 inhibitor geldanamycin. Geldanamycin effectively promotes the degradation of CRAF, thereby revealing a potential therapeutic strategy to overcome resistance to RAF inhibition in a subset of BRAF mutant tumors.
Figures
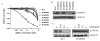

References
-
- Sharma SV, Settleman J. Oncogene addiction: Setting the stage for molecularly targeted cancer therapy. Genes Dev. 2007;21(24):3214–31. - PubMed
-
- Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344(14):1031–7. - PubMed
-
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–39. - PubMed
-
- Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–500. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

