T regulatory type 1 cells in squamous cell carcinoma of the head and neck: mechanisms of suppression and expansion in advanced disease
- PMID: 18559587
- PMCID: PMC3708468
- DOI: 10.1158/1078-0432.CCR-07-5126
T regulatory type 1 cells in squamous cell carcinoma of the head and neck: mechanisms of suppression and expansion in advanced disease
Abstract
Purpose: Regulatory T cells play a major role in tumor escape from immunosurveillance. T regulatory cells type 1 (Tr1), a subset of regulatory T cells present in the tumor and peripheral circulation of patients with head and neck squamous cell carcinoma (HNSCC), mediate immune suppression and might contribute to tumor progression.
Experimental design: CD4+CD25-T cells were isolated from peripheral blood mononuclear cells (PBMC) or tumor-infiltrating lymphocytes (TIL) of 26 HNSCC patients and 10 normal controls. The Tr1 cell phenotype was determined before and after culture in the presence of interleukin (IL)-2, IL-10, and IL-15, each at 10 to 20 IU/mL. Suppression was measured in carboxyfluorescein diacetate succinimidyl ester-based proliferation assays with or without neutralizing anti-IL-10 or anti-transforming growth factor-beta1 (TGF-beta1) monoclonal antibodies in Transwell systems. ELISA was used to define the Tr1 cytokine profile.
Results: Tr1 cells originate from CD4(+)CD25(-) precursors present in TIL and PBMC of HNSCC patients. Cytokine-driven ex vivo expansion of Tr1 precursors yielded CD4+CD25-Foxp3lowCD132+IL-10+TGF-beta1+ populations that mediated higher suppression than Tr1 cells of normal controls (P < 0.0001). Tr1 cells suppressed proliferation of autologous responders via IL-10 and TGF-beta1 secretion. Expression of these cytokines was higher in TIL-derived than PBMC-derived Tr1 cells (P < 0.0001). The Tr1 cell frequency and suppressor function were significantly higher in patients presenting with advanced than early disease stages and in patients "cured" by oncologic therapies than in those with active disease.
Conclusions: In HNSCC, Tr1 cell generation is promoted at the tumor site. Tr1 cells use TGF-beta and IL-10 to mediate suppression. They expand during disease progression and also following cancer therapy in patients with no evident disease.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures
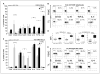
 ) or from tumor tissue of HNSCC patients (■). B, flow cytometry dot blots showing relative expression of selected markers on sorted fresh PBMC (top row) or tumor-derived cells (TIL; bottom row). Data are representative for CD4+CD25− cells sorted from lymphocytes of different HNSCC patients. C, phenotypic analysis of single-cell sorted CD4+CD25− T cells obtained from fresh PBMC versus TIL from HNSCC patients and cultured in the presence of “Tr1 cytokines” for 10 d. A and C, are mean ± SD percent positive cells from experiments done with cells of 26 different HNSCC patients. *, P < 0.0006; **, P < 0.0001. Multicolor flow cytometry was done with gates set on CD3+CD4+ T cells. D, box plots show differences in MFI in IL-10 or TGF-β1 expression in PBMC versus TIL. E, representative flow cytometry dot blots showing relative expression of selected markers on CD4+CD25− lymphocytes after culture in the presence of “Tr1 cytokines” for 10 d.
) or from tumor tissue of HNSCC patients (■). B, flow cytometry dot blots showing relative expression of selected markers on sorted fresh PBMC (top row) or tumor-derived cells (TIL; bottom row). Data are representative for CD4+CD25− cells sorted from lymphocytes of different HNSCC patients. C, phenotypic analysis of single-cell sorted CD4+CD25− T cells obtained from fresh PBMC versus TIL from HNSCC patients and cultured in the presence of “Tr1 cytokines” for 10 d. A and C, are mean ± SD percent positive cells from experiments done with cells of 26 different HNSCC patients. *, P < 0.0006; **, P < 0.0001. Multicolor flow cytometry was done with gates set on CD3+CD4+ T cells. D, box plots show differences in MFI in IL-10 or TGF-β1 expression in PBMC versus TIL. E, representative flow cytometry dot blots showing relative expression of selected markers on CD4+CD25− lymphocytes after culture in the presence of “Tr1 cytokines” for 10 d.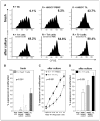
 ) or TIL (■). C, titration of Tr1 cells (suppressor cells) derived from PBMC or TIL into CFSE-labeled autologous proliferating responder cell cultures. Suppression by Tr1 cells expanded from TIL (■) or from PBMC (◆) of proliferating responder cells is shown at various ratios. D, percentages of suppression of proliferation in single-cell sorted CFSE-labeled CD4+CD25− responder cells by autologous suppressor cells cultured with “Tr1 cytokines” for 10 d. Suppressor cell/responder cell ratio was 1:1. Mean ± SD percent suppression for cocultures done with cells of 10 normal controls and 26 HNSCC patients.
) or TIL (■). C, titration of Tr1 cells (suppressor cells) derived from PBMC or TIL into CFSE-labeled autologous proliferating responder cell cultures. Suppression by Tr1 cells expanded from TIL (■) or from PBMC (◆) of proliferating responder cells is shown at various ratios. D, percentages of suppression of proliferation in single-cell sorted CFSE-labeled CD4+CD25− responder cells by autologous suppressor cells cultured with “Tr1 cytokines” for 10 d. Suppressor cell/responder cell ratio was 1:1. Mean ± SD percent suppression for cocultures done with cells of 10 normal controls and 26 HNSCC patients.
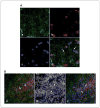


References
-
- Lang S, Wollenberg B, Dellian M, et al. Clinical and epidemiological data of patients with malignomas of the head and neck. Laryngorhinootologie. 2002;81:499–508. - PubMed
-
- Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. N Engl J Med. 2001;345:1890–900. - PubMed
-
- Chin D, Boyle GM, Porceddu S, Theile DR, Parsons PG, Coman WB. Head and neck cancer: past, present and future. Expert Rev Anticancer Ther. 2006;6:1111–8. - PubMed
-
- Young MR. Protective mechanisms of head and neck squamous cell carcinomas from immune assault. Head Neck. 2006;28:462–70. - PubMed
-
- Whiteside TL. Immunobiology of head and neck cancer. Cancer Metastasis Rev. 2005;24:95–105. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

