Phosphodiesterase 11A (PDE11A) and genetic predisposition to adrenocortical tumors
- PMID: 18559625
- PMCID: PMC3134879
- DOI: 10.1158/1078-0432.CCR-08-0106
Phosphodiesterase 11A (PDE11A) and genetic predisposition to adrenocortical tumors
Abstract
Purpose: We have reported previously nonsense inactivating mutations of the phosphodiesterase 11A (PDE11A) gene in patients with micronodular adrenocortical hyperplasia and Cushing syndrome. The aim of this study is to investigate the presence of somatic or germ-line PDE11A mutations in various types of adrenocortical tumors: ACTH-independent macronodular adrenocortical hyperplasia (AIMAH), adrenocortical adenoma (ACA), and adrenocortical cancer (ACC).
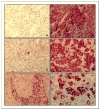
Experimental design: PDE11A was sequenced in 117 adrenocortical tumors and 192 controls subjects; immunohistochemistry for PDE11A and tumor cyclic AMP levels were studied in a subgroup of adrenocortical tumors.
Results: One PDE11A inactivating mutation (R307X) was found in one ACA, 22 germ-line missense variants (18.8%) were found in adrenocortical tumors, and only 11 missense variants (5.7%) were found in controls. By comparing the common mutations, a higher frequency of mutations in adrenocortical tumors than in age/sex-matched controls were observed [16% versus 10% in ACC, 19% versus 10% in ACA, and 24% versus 9% in AIMAH; odds ratio (OR), 3.53; P = 0.05]. Somatic DNA from adrenocortical tumors with missense variants showed a wild-type allelic loss. A significant difference between ACC and controls was observed for a polymorphism in exon 6 (E421E; OR, 2.1; P = 0.03) and three associated polymorphisms located in intron 10-exon 11-intron 11 (OR, 0.5; P = 0.01). In AIMAH/ACA, cyclic AMP levels were higher than in normal adrenals and decreased PDE11A immunostaining was present in adrenocortical tumors with PDE11A variants.
Conclusions: The present investigation of a large cohort of adrenocortical tumors suggests that PDE11A sequence defects predispose to a variety of lesions (beyond micronodular adrenocortical hyperplasia) and may contribute to the development of these tumors in the general population.
Figures



Similar articles
-
Phosphodiesterase 11A (PDE11A) gene defects in patients with acth-independent macronodular adrenal hyperplasia (AIMAH): functional variants may contribute to genetic susceptibility of bilateral adrenal tumors.J Clin Endocrinol Metab. 2012 Nov;97(11):E2063-9. doi: 10.1210/jc.2012-2275. Epub 2012 Sep 20. J Clin Endocrinol Metab. 2012. PMID: 22996146 Free PMC article.
-
Adrenal hyperplasia and adenomas are associated with inhibition of phosphodiesterase 11A in carriers of PDE11A sequence variants that are frequent in the population.Cancer Res. 2006 Dec 15;66(24):11571-5. doi: 10.1158/0008-5472.CAN-06-2914. Cancer Res. 2006. PMID: 17178847
-
Phosphodiesterase 11A expression in the adrenal cortex, primary pigmented nodular adrenocortical disease, and other corticotropin-independent lesions.Horm Metab Res. 2008 May;40(5):347-53. doi: 10.1055/s-2008-1076694. Horm Metab Res. 2008. PMID: 18491255 Free PMC article.
-
Alterations of Phosphodiesterases in Adrenocortical Tumors.Front Endocrinol (Lausanne). 2016 Aug 30;7:111. doi: 10.3389/fendo.2016.00111. eCollection 2016. Front Endocrinol (Lausanne). 2016. PMID: 27625633 Free PMC article. Review.
-
New genes and/or molecular pathways associated with adrenal hyperplasias and related adrenocortical tumors.Mol Cell Endocrinol. 2009 Mar 5;300(1-2):152-7. doi: 10.1016/j.mce.2008.11.010. Epub 2008 Nov 21. Mol Cell Endocrinol. 2009. PMID: 19063937 Free PMC article. Review.
Cited by
-
Frequent phosphodiesterase 11A gene (PDE11A) defects in patients with Carney complex (CNC) caused by PRKAR1A mutations: PDE11A may contribute to adrenal and testicular tumors in CNC as a modifier of the phenotype.J Clin Endocrinol Metab. 2011 Jan;96(1):E208-14. doi: 10.1210/jc.2010-1704. Epub 2010 Nov 3. J Clin Endocrinol Metab. 2011. PMID: 21047926 Free PMC article.
-
ARMC5 variants in PRKAR1A-mutated patients modify cortisol levels and Cushing's syndrome.Endocr Relat Cancer. 2020 Sep;27(9):509-517. doi: 10.1530/ERC-20-0273. Endocr Relat Cancer. 2020. PMID: 32638579 Free PMC article.
-
p54nrb/NONO regulates cyclic AMP-dependent glucocorticoid production by modulating phosphodiesterase mRNA splicing and degradation.Mol Cell Biol. 2015 Apr;35(7):1223-37. doi: 10.1128/MCB.00993-14. Epub 2015 Jan 20. Mol Cell Biol. 2015. PMID: 25605330 Free PMC article.
-
Primary bilateral macronodular adrenal hyperplasia: definitely a genetic disease.Nat Rev Endocrinol. 2022 Nov;18(11):699-711. doi: 10.1038/s41574-022-00718-y. Epub 2022 Aug 3. Nat Rev Endocrinol. 2022. PMID: 35922573 Review.
-
The Many Faces of Primary Aldosteronism and Cushing Syndrome: A Reflection of Adrenocortical Tumor Heterogeneity.Front Med (Lausanne). 2018 Mar 12;5:54. doi: 10.3389/fmed.2018.00054. eCollection 2018. Front Med (Lausanne). 2018. PMID: 29594118 Free PMC article. Review.
References
-
- Sidhu S, Sywak M, Robinson B, Delbridge L. Adrenocortical cancer: recent clinical and molecular advances. Curr Opin Oncol. 2004;16:13–8. - PubMed
-
- Libe R, Bertherat J. Molecular genetics of adrenocortical tumours, from familial to sporadic diseases. Eur J Endocrinol. 2005;153:477–87. - PubMed
-
- Beuschlein F, Reincke M. Adrenocortical tumorigenesis. Ann N Y Acad Sci. 2006;1088:319–34. - PubMed
-
- Libe R, Fratticci A, Bertherat J. Adrenocortical cancer: pathophysiology and clinical management. Endocr Relat Cancer. 2007;14:13–28. - PubMed
-
- Stratakis CA, Boikos SA. Genetics of adrenal tumors associated with Cushing’s syndrome: a new classification for bilateral adrenocortical hyperplasias. Nat Clin Pract Endocrinol Metab. 2007;3:748–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

