Molecular mechanism of hepatitis C virus replicon variants with reduced susceptibility to a benzofuran inhibitor, HCV-796
- PMID: 18559648
- PMCID: PMC2533464
- DOI: 10.1128/AAC.00238-08
Molecular mechanism of hepatitis C virus replicon variants with reduced susceptibility to a benzofuran inhibitor, HCV-796
Abstract
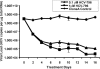
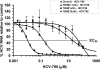
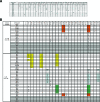
HCV-796 selectively inhibits hepatitis C virus (HCV) NS5B RNA-dependent RNA polymerase. In hepatoma cells containing a genotype 1b HCV replicon, HCV-796 reduced HCV RNA levels by 3 to 4 log(10) HCV copies/mug total RNA (the concentration of the compound that inhibited 50% of the HCV RNA level was 9 nM). Cells bearing replicon variants with reduced susceptibility to HCV-796 were generated in the presence of HCV-796, followed by G418 selection. Sequence analysis of the NS5B gene derived from the replicon variants revealed several amino acid changes within 5 A of the drug-binding pocket. Specifically, mutations were observed at Leu314, Cys316, Ile363, Ser365, and Met414 of NS5B, which directly interact with HCV-796. The impacts of the amino acid substitutions on viral fitness and drug susceptibility were examined in recombinant replicons and NS5B enzymes with the single-amino-acid mutations. The replicon variants were 10- to 1,000-fold less efficient in forming colonies in cells than the wild-type replicon; the S365L variant failed to establish a stable cell line. Other variants (L314F, I363V, and M414V) had four- to ninefold-lower steady-state HCV RNA levels. Reduced binding affinity with HCV-796 was demonstrated in an enzyme harboring the C316Y mutation. The effects of these resistance mutations were structurally rationalized using X-ray crystallography data. While different levels of resistance to HCV-796 were observed in the replicon and enzyme variants, these variants retained their susceptibilities to pegylated interferon, ribavirin, and other HCV-specific inhibitors. The combined virological, biochemical, biophysical, and structural approaches revealed the mechanism of resistance in the variants selected by the potent polymerase inhibitor HCV-796.
Figures

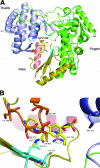

Similar articles
-
Selected replicon variants with low-level in vitro resistance to the hepatitis C virus NS5B polymerase inhibitor PSI-6130 lack cross-resistance with R1479.Antimicrob Agents Chemother. 2008 Dec;52(12):4356-69. doi: 10.1128/AAC.00444-08. Epub 2008 Oct 6. Antimicrob Agents Chemother. 2008. PMID: 18838588 Free PMC article.
-
Selection of replicon variants resistant to ACH-806, a novel hepatitis C virus inhibitor with no cross-resistance to NS3 protease and NS5B polymerase inhibitors.Antimicrob Agents Chemother. 2008 Jun;52(6):2043-52. doi: 10.1128/AAC.01548-07. Epub 2008 Apr 14. Antimicrob Agents Chemother. 2008. PMID: 18411324 Free PMC article.
-
Selection and characterization of hepatitis C virus replicons dually resistant to the polymerase and protease inhibitors HCV-796 and boceprevir (SCH 503034).Antimicrob Agents Chemother. 2009 Feb;53(2):401-11. doi: 10.1128/AAC.01081-08. Epub 2008 Oct 20. Antimicrob Agents Chemother. 2009. PMID: 18936191 Free PMC article.
-
Hepatitis C virus resistance to protease inhibitors.J Hepatol. 2011 Jul;55(1):192-206. doi: 10.1016/j.jhep.2011.01.011. Epub 2011 Feb 1. J Hepatol. 2011. PMID: 21284949 Review.
-
HCV antiviral resistance: the impact of in vitro studies on the development of antiviral agents targeting the viral NS5B polymerase.Antivir Chem Chemother. 2005;16(4):225-45. doi: 10.1177/095632020501600403. Antivir Chem Chemother. 2005. PMID: 16130521 Review.
Cited by
-
Genotype and subtype profiling of PSI-7977 as a nucleotide inhibitor of hepatitis C virus.Antimicrob Agents Chemother. 2012 Jun;56(6):3359-68. doi: 10.1128/AAC.00054-12. Epub 2012 Mar 19. Antimicrob Agents Chemother. 2012. PMID: 22430955 Free PMC article.
-
Therapeutic implications of hepatitis C virus resistance to antiviral drugs.Therap Adv Gastroenterol. 2009 Jul;2(4):205-19. doi: 10.1177/1756283X09336045. Therap Adv Gastroenterol. 2009. PMID: 21180544 Free PMC article.
-
Inhibition of hepatitis C virus replicon RNA synthesis by PSI-352938, a cyclic phosphate prodrug of β-D-2'-deoxy-2'-α-fluoro-2'-β-C-methylguanosine.Antimicrob Agents Chemother. 2011 Jun;55(6):2566-75. doi: 10.1128/AAC.00032-11. Epub 2011 Mar 28. Antimicrob Agents Chemother. 2011. PMID: 21444700 Free PMC article.
-
Slow binding inhibition and mechanism of resistance of non-nucleoside polymerase inhibitors of hepatitis C virus.J Biol Chem. 2009 Jun 5;284(23):15517-29. doi: 10.1074/jbc.M808889200. Epub 2009 Feb 26. J Biol Chem. 2009. PMID: 19246450 Free PMC article.
-
Preclinical characterization of GSK2336805, a novel inhibitor of hepatitis C virus replication that selects for resistance in NS5A.Antimicrob Agents Chemother. 2014;58(1):38-47. doi: 10.1128/AAC.01363-13. Epub 2013 Oct 14. Antimicrob Agents Chemother. 2014. PMID: 24126581 Free PMC article.
References
-
- Afdhal, N., E. Godofsky, J. Dienstag, V. Rustgi, L. Schick, D. McEnlry, X. S. Zhou, G. Chao, C. Fang, B. Fielman, and M. Myers. 2004. Final Phase I/II trial results for NM283, a new polymerase inhibitor for hepatitis C: Antiviral efficacy and tolerance in patients with HCV-1 infection, including previous interferon failures. Hepatology 40(Suppl. 1):726. (Abstract.)
-
- Akahane, Y., M. Sakamoto, Y. Miyazaki, S. Okada, T. Inoue, M. Ukita, H. Okamoto, Y. Miyakawa, and M. Mayumi. 1999. Effect of interferon on a nonenveloped DNA virus (TT virus) associated with acute and chronic hepatitis of unknown etiology. J. Med. Virol. 58:196-200. - PubMed
-
- Blight, K. J., A. A. Kolykhalov, and C. M. Rice. 2000. Efficient initiation of HCV RNA replication in cell culture. Science 290:1972-1974. - PubMed
-
- Carroll, S. S., J. E. Tomassini, M. Bosserman, K. Getty, M. W. Stahlhut, A. B. Eldrup, B. Bhat, D. Hall, A. L. Simcoe, R. LaFemina, C. A. Rutkowski, B. Wolanski, Z. C. Yang, G. Migliaccio, R. De Francesco, L. C. Kuo, M. MacCoss, and D. B. Olsen. 2003. Inhibition of hepatitis C virus RNA replication by 2′-modified nucleoside analogs. J. Biol. Chem. 278:11979-11984. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

