Cytokine secretion requires phosphatidylcholine synthesis
- PMID: 18559668
- PMCID: PMC2426940
- DOI: 10.1083/jcb.200706152
Cytokine secretion requires phosphatidylcholine synthesis
Abstract
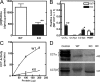
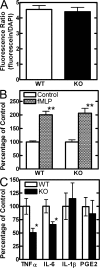
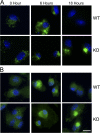
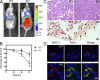
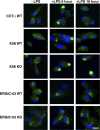
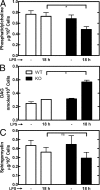
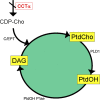
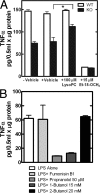
Choline cytidylyltransferase (CCT) is the rate-limiting enzyme in the phosphatidylcholine biosynthetic pathway. Here, we demonstrate that CCT alpha-mediated phosphatidylcholine synthesis is required to maintain normal Golgi structure and function as well as cytokine secretion from the Golgi complex. CCT alpha is localized to the trans-Golgi region and its expression is increased in lipopolysaccharide (LPS)-stimulated wild-type macrophages. Although LPS triggers transient reorganization of Golgi morphology in wild-type macrophages, similar structural alterations persist in CCT alpha-deficient cells. Pro-tumor necrosis factor alpha and interleukin-6 remain lodged in the secretory compartment of CCT alpha-deficient macrophages after LPS stimulation. However, the lysosomal-mediated secretion pathways for interleukin-1 beta secretion and constitutive apolipoprotein E secretion are unaltered. Exogenous lysophosphatidylcholine restores LPS-stimulated secretion from CCT alpha-deficient cells, and elevated diacylglycerol levels alone do not impede secretion of pro-tumor necrosis factor alpha or interleukin-6. These results identify CCT alpha as a key component in membrane biogenesis during LPS-stimulated cytokine secretion from the Golgi complex.
Figures

References
-
- Altan-Bonnet, N., R. Sougrat, and J. Lippincott-Schwartz. 2004. Molecular basis for Golgi maintenance and biogenesis. Curr. Opin. Cell Biol. 16:364–372. - PubMed
-
- Baburina, I., and S. Jackowski. 1998. Apoptosis triggered by 1-O-Octadecyl-2-O-methyl-rac-glycero-3-phosphocholine is prevented by increased expression of CTP:phosphocholine cytidylyltransferase. J. Biol. Chem. 273:2169–2173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

