Regulation of RhoA-dependent ROCKII activation by Shp2
- PMID: 18559669
- PMCID: PMC2426933
- DOI: 10.1083/jcb.200710187
Regulation of RhoA-dependent ROCKII activation by Shp2
Abstract
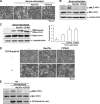
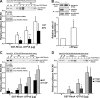
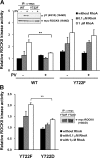
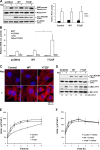
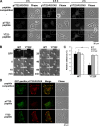
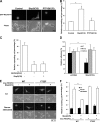
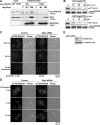
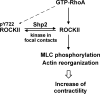
Contractile forces mediated by RhoA and Rho kinase (ROCK) are required for a variety of cellular processes, including cell adhesion. In this study, we show that RhoA-dependent ROCKII activation is negatively regulated by phosphorylation at a conserved tyrosine residue (Y722) in the coiled-coil domain of ROCKII. Tyrosine phosphorylation of ROCKII is increased with cell adhesion, and loss of Y722 phosphorylation delays adhesion and spreading on fibronectin, suggesting that this modification is critical for restricting ROCKII-mediated contractility during these processes. Further, we provide evidence that Shp2 mediates dephosphorylation of ROCKII and, therefore, regulates RhoA-induced cell rounding, indicating that Shp2 couples with RhoA signaling to control ROCKII activation during deadhesion. Thus, reversible tyrosine phosphorylation confers an additional layer of control to fine-tune RhoA-dependent activation of ROCKII.
Figures

Similar articles
-
ROCKII Ser1366 phosphorylation reflects the activation status.Biochem J. 2012 Apr 1;443(1):145-51. doi: 10.1042/BJ20111839. Biochem J. 2012. PMID: 22273145
-
Src-dependent phosphorylation of ROCK participates in regulation of focal adhesion dynamics.J Cell Sci. 2010 Oct 1;123(Pt 19):3368-77. doi: 10.1242/jcs.071555. Epub 2010 Sep 7. J Cell Sci. 2010. PMID: 20826462
-
Angiotensin II type-1 receptor regulates RhoA and Rho-kinase/ROCK activation via multiple mechanisms. Focus on "Angiotensin II induces RhoA activation through SHP2-dependent dephosphorylation of the RhoGAP p190A in vascular smooth muscle cells".Am J Physiol Cell Physiol. 2009 Nov;297(5):C1059-61. doi: 10.1152/ajpcell.00399.2009. Epub 2009 Sep 9. Am J Physiol Cell Physiol. 2009. PMID: 19741194 Free PMC article. No abstract available.
-
Abnormal activation of Ras/Raf/MAPK and RhoA/ROCKII signalling pathways in eutopic endometrial stromal cells of patients with endometriosis.Hum Reprod. 2011 Apr;26(4):885-97. doi: 10.1093/humrep/der010. Epub 2011 Feb 7. Hum Reprod. 2011. PMID: 21303778
-
Made to measure - keeping Rho kinase at a distance.Small GTPases. 2016 Apr 2;7(2):82-92. doi: 10.1080/21541248.2016.1173770. Epub 2016 Apr 12. Small GTPases. 2016. PMID: 27070834 Free PMC article. Review.
Cited by
-
The Cell Adhesion Molecule Necl-4/CADM4 Serves as a Novel Regulator for Contact Inhibition of Cell Movement and Proliferation.PLoS One. 2015 Apr 20;10(4):e0124259. doi: 10.1371/journal.pone.0124259. eCollection 2015. PLoS One. 2015. PMID: 25893857 Free PMC article.
-
Ser1333 phosphorylation indicates ROCKI activation.J Biomed Sci. 2013 Oct 29;20(1):83. doi: 10.1186/1423-0127-20-83. J Biomed Sci. 2013. PMID: 24168723 Free PMC article.
-
The tyrosine phosphatase SHP2 regulates focal adhesion kinase to promote EGF-induced lamellipodia persistence and cell migration.Mol Cancer Res. 2013 Jun;11(6):651-64. doi: 10.1158/1541-7786.MCR-12-0578. Epub 2013 Mar 19. Mol Cancer Res. 2013. PMID: 23512980 Free PMC article.
-
From mechanical force to RhoA activation.Biochemistry. 2012 Sep 25;51(38):7420-32. doi: 10.1021/bi300758e. Epub 2012 Sep 10. Biochemistry. 2012. PMID: 22931484 Free PMC article. Review.
-
The on-off relationship of Rho and Rac during integrin-mediated adhesion and cell migration.Small GTPases. 2014;5:e27958. doi: 10.4161/sgtp.27958. Epub 2014 Mar 7. Small GTPases. 2014. PMID: 24607953 Free PMC article. Review.
References
-
- Amano, M., M. Ito, K. Kimura, Y. Fukata, K. Chihara, T. Nakano, Y. Matsuura, and K. Kaibuchi. 1996. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J. Biol. Chem. 271:20246–20249. - PubMed
-
- Amano, M., K. Chihara, N. Nakamura, T. Kaneko, Y. Matsuura, and K. Kaibuchi. 1999. The COOH terminus of Rho-kinase negatively regulates rho-kinase activity. J. Biol. Chem. 274:32418–32424. - PubMed
-
- Amano, M., Y. Fukata, H. Shimokawa, and K. Kaibuchi. 2000. Purification and in vitro activity of Rho-associated kinase. Methods Enzymol. 325:149–155. - PubMed
-
- Arnaud, M., R. Mzali, F. Gesbert, C. Crouin, C. Guenzi, C. Vermot-Desroches, J. Wijdenes, G. Courtois, O. Bernard, and J. Bertoglio. 2004. Interaction of the tyrosine phosphatase SHP-2 with Gab2 regulates Rho-dependent activation of the c-fos serum response element by interleukin-2. Biochem. J. 382:545–556. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

