A neuron-specific cytoplasmic dynein isoform preferentially transports TrkB signaling endosomes
- PMID: 18559670
- PMCID: PMC2426944
- DOI: 10.1083/jcb.200803150
A neuron-specific cytoplasmic dynein isoform preferentially transports TrkB signaling endosomes
Abstract
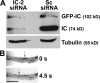
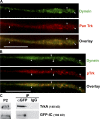
Cytoplasmic dynein is the multisubunit motor protein for retrograde movement of diverse cargoes to microtubule minus ends. Here, we investigate the function of dynein variants, defined by different intermediate chain (IC) isoforms, by expressing fluorescent ICs in neuronal cells. Green fluorescent protein (GFP)-IC incorporates into functional dynein complexes that copurify with membranous organelles. In living PC12 cell neurites, GFP-dynein puncta travel in both the anterograde and retrograde directions. In cultured hippocampal neurons, neurotrophin receptor tyrosine kinase B (TrkB) signaling endosomes are transported by cytoplasmic dynein containing the neuron-specific IC-1B isoform and not by dynein containing the ubiquitous IC-2C isoform. Similarly, organelles containing TrkB isolated from brain by immunoaffinity purification also contain dynein with IC-1 but not IC-2 isoforms. These data demonstrate that the IC isoforms define dynein populations that are selectively recruited to transport distinct cargoes.
Figures







References
-
- Arevalo, J.C., D.B. Pereira, H. Yano, K.K. Teng, and M.V. Chao. 2006. Identification of a switch in neurotrophin signaling by selective tyrosine phosphorylation. J. Biol. Chem. 281:1001–1007. - PubMed
-
- Bhattacharyya, A., F.L. Watson, S.L. Pomeroy, Y.Z. Zhang, C.D. Stiles, and R.A. Segal. 2002. High-resolution imaging demonstrates dynein-based vesicular transport of activated Trk receptors. J. Neurobiol. 51:302–312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

