Analysis of the spatial and temporal characteristics of platelet-delivered factor VIII-based clots
- PMID: 18559671
- PMCID: PMC2515146
- DOI: 10.1182/blood-2008-04-152959
Analysis of the spatial and temporal characteristics of platelet-delivered factor VIII-based clots
Abstract
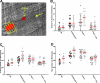
Normally factor (F) VIII is not expressed in megakaryocytes, but when human FVIII was transgenically expressed in murine megakaryocytes, it was stored in platelet alpha-granules and released at sites of injury. This platelet FVIII (pFVIII) is effective in correcting hemostasis, even in the presence of circulating inhibitors, so it offers a potential gene therapy strategy for hemophilia A. To understand clot development by pFVIII, we have examined clot response to laser injury in both cremaster arterioles and venules in FVIII(null) mice either infused with FVIII or transgenic for pFVIII. In both sets of vessels, pFVIII is at least as effective as infused FVIII. However, there are temporal and spatial differences in fibrin and platelet accumulation within clots depending on how FVIII is delivered. These differences may be related to the temporal and spatial distribution of the alpha-granular-released FVIII within the developing clot, and may explain the increased frequency and size of embolic events seen with pFVIII. These observations may not only have implications for the use of pFVIII in gene therapy for hemophilia A, but may also have physiologic consequences, explaining why many procoagulant factors are delivered both in the plasma and in platelet alpha-granules.
Figures


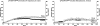


References
-
- Yarovoi H, Kufrin D, Eslin DE, et al. Factor VIII ectopically expressed in platelets: efficacy in hemophilia A treatment. Blood. 2003;102:4006–4013. - PubMed
-
- Yarovoi H, Nurden AT, Montgomery RR, Nurden P, Poncz M. Intracellular interaction of von Willebrand factor and factor VIII depends on cellular context: lessons from platelet-expressed factor VIII. Blood. 2005;105:4674–4676. - PubMed
-
- Gewirtz J, Thornton M, Rauova L, Poncz M. Platelet-delivered factor VIII provides limited resistance to anti-factor VIII inhibitors. J Thromb Haemost. 2008 Apr 23; [Epub ahead of print] - PubMed
-
- Dasgupta S, Navarrete AM, Delignat S, et al. Immune response against therapeutic factor VIII in hemophilia A patients–a survey of probable risk factors. Immunol Lett. 2007;110:23–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

