Thermal ablation of tumor cells with antibody-functionalized single-walled carbon nanotubes
- PMID: 18559847
- PMCID: PMC2438394
- DOI: 10.1073/pnas.0803557105
Thermal ablation of tumor cells with antibody-functionalized single-walled carbon nanotubes
Abstract
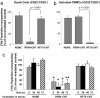
Single-walled carbon nanotubes (CNTs) emit heat when they absorb energy from near-infrared (NIR) light. Tissue is relatively transparent to NIR, which suggests that targeting CNTs to tumor cells, followed by noninvasive exposure to NIR light, will ablate tumors within the range of NIR. In this study, we demonstrate the specific binding of antibody-coupled CNTs to tumor cells in vitro, followed by their highly specific ablation with NIR light. Biotinylated polar lipids were used to prepare stable, biocompatible, noncytotoxic CNT dispersions that were then attached to one of two different neutralite avidin-derivatized mAbs directed against either human CD22 or CD25. CD22(+)CD25(-) Daudi cells bound only CNTs coupled to the anti-CD22 mAb; CD22(-)CD25(+) activated peripheral blood mononuclear cells bound only to the CNTs coupled to the anti-CD25 mAb. Most importantly, only the specifically targeted cells were killed after exposure to NIR light.
Conflict of interest statement
Conflict of interest statement: E.S.V., R.K.D., P.P., and I.H.M. are affiliated with Medical Nanotechnologies, Inc. E.S.V. is a coinventor on an issued patent encompassing this work.
Figures
Similar articles
-
Specific thermal ablation of tumor cells using single-walled carbon nanotubes targeted by covalently-coupled monoclonal antibodies.Int J Cancer. 2009 Dec 15;125(12):2970-7. doi: 10.1002/ijc.24659. Int J Cancer. 2009. PMID: 19536775
-
The importance of cellular internalization of antibody-targeted carbon nanotubes in the photothermal ablation of breast cancer cells.Nanotechnology. 2011 Mar 4;22(9):095101. doi: 10.1088/0957-4484/22/9/095101. Epub 2011 Jan 24. Nanotechnology. 2011. PMID: 21258147
-
In vitro photothermal destruction of neuroblastoma cells using carbon nanotubes conjugated with GD2 monoclonal antibody.Nanotechnology. 2009 Aug 5;20(31):315101. doi: 10.1088/0957-4484/20/31/315101. Epub 2009 Jul 13. Nanotechnology. 2009. PMID: 19597244
-
Single-walled carbon nanotubes-111In-1,4,7,10-tetraazacyclododecane-N,N',N'',N'''-tetraacetic acid-rituximab.2007 Aug 21 [updated 2007 Dec 21]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2007 Aug 21 [updated 2007 Dec 21]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641770 Free Books & Documents. Review.
-
Carbon nanotubes for delivery of small molecule drugs.Adv Drug Deliv Rev. 2013 Dec;65(15):1964-2015. doi: 10.1016/j.addr.2013.08.005. Epub 2013 Aug 14. Adv Drug Deliv Rev. 2013. PMID: 23954402 Review.
Cited by
-
Upconverting organic dye doped core-shell nano-composites for dual-modality NIR imaging and photo-thermal therapy.Theranostics. 2013 Mar 21;3(4):267-74. doi: 10.7150/thno.5226. Print 2013. Theranostics. 2013. PMID: 23606913 Free PMC article.
-
Recent Insights into NIR-Light-Responsive Materials for Photothermal Cell Treatments.Nanomaterials (Basel). 2022 Sep 23;12(19):3318. doi: 10.3390/nano12193318. Nanomaterials (Basel). 2022. PMID: 36234446 Free PMC article. Review.
-
Advances in the field of nanooncology.BMC Med. 2010 Dec 13;8:83. doi: 10.1186/1741-7015-8-83. BMC Med. 2010. PMID: 21144040 Free PMC article. Review.
-
Effect of a session of intensive exercise with ginseng supplementation on histone H3 protein methylation of skeletal muscle of nonathlete men.Mol Genet Genomic Med. 2019 May;7(5):e651. doi: 10.1002/mgg3.651. Epub 2019 Mar 28. Mol Genet Genomic Med. 2019. PMID: 30920174 Free PMC article. Clinical Trial.
-
Enabling individualized therapy through nanotechnology.Pharmacol Res. 2010 Aug;62(2):57-89. doi: 10.1016/j.phrs.2009.12.011. Epub 2010 Jan 5. Pharmacol Res. 2010. PMID: 20045055 Free PMC article. Review.
References
-
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. - PubMed
-
- Weiner LM, Adams GP. New approaches to antibody therapy. Oncogene. 2000;19:6144–6151. - PubMed
-
- von Mehren AG, Weiner LM. Monoclonal antibody therapy for cancer. Annu Rev Med. 2003;54:343–369. - PubMed
-
- Bross PF, et al. Approval summary: Gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin Cancer Res. 2001;7:1490–1496. - PubMed
-
- Sharkey RM, Burton J, Goldenberg DM. Radioimmunotherapy of non-Hodgkin's lymphoma: A critical appraisal. Exp Rev Clin Immunol. 2005;1:47–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

