Thrombogenic collagen-mimetic peptides: Self-assembly of triple helix-based fibrils driven by hydrophobic interactions
- PMID: 18559857
- PMCID: PMC2438399
- DOI: 10.1073/pnas.0800291105
Thrombogenic collagen-mimetic peptides: Self-assembly of triple helix-based fibrils driven by hydrophobic interactions
Abstract
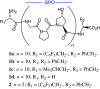
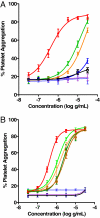
Collagens are integral structural proteins in animal tissues and play key functional roles in cellular modulation. We sought to discover collagen model peptides (CMPs) that would form triple helices and self-assemble into supramolecular fibrils exhibiting collagen-like biological activity without preorganizing the peptide chains by covalent linkages. This challenging objective was accomplished by placing aromatic groups on the ends of a representative 30-mer CMP, (GPO)(10), as with l-phenylalanine and l-pentafluorophenylalanine in 32-mer 1a. Computational studies on homologous 29-mers 1a'-d' (one less GPO), as pairs of triple helices interacting head-to-tail, yielded stabilization energies in the order 1a' > 1b' > 1c' > 1d', supporting the hypothesis that hydrophobic aromatic groups can drive CMP self-assembly. Peptides 1a-d were studied comparatively relative to structural properties and ability to stimulate human platelets. Although each 32-mer formed stable triple helices (CD) spectroscopy, only 1a and 1b self-assembled into micrometer-scale fibrils. Light microscopy images for 1a depicted long collagen-like fibrils, whereas images for 1d did not. Atomic force microscopy topographical images indicated that 1a and 1b self-organize into microfibrillar species, whereas 1c and 1d do not. Peptides 1a and 1b induced the aggregation of human blood platelets with a potency similar to type I collagen, whereas 1c was much less effective, and 1d was inactive (EC(50) potency: 1a/1b >> 1c > 1d). Thus, 1a and 1b spontaneously self-assemble into thrombogenic collagen-mimetic materials because of hydrophobic aromatic interactions provided by the special end-groups. These findings have important implications for the design of biofunctional CMPs.
Conflict of interest statement
Conflict of interest statement: M.A.C, W.A.K., C.C., J.G.V., H.R.A., K.M.B., C.A.M, U.S., M.B., A.M. E.L., and B.E.M. conducted the studies described herein while employed by a commercial enterprise.
Figures



Similar articles
-
Effects of glycosylated (2S,4R)-hydroxyproline on the stability and assembly of collagen triple helices.Amino Acids. 2016 Dec;48(12):2765-2772. doi: 10.1007/s00726-016-2312-2. Epub 2016 Aug 13. Amino Acids. 2016. PMID: 27522650
-
Supramolecular Nanofibers from Collagen-Mimetic Peptides Bearing Various Aromatic Groups at N-Termini via Hierarchical Self-Assembly.Int J Mol Sci. 2021 Apr 26;22(9):4533. doi: 10.3390/ijms22094533. Int J Mol Sci. 2021. PMID: 33926094 Free PMC article.
-
The predominant roles of the sequence periodicity in the self-assembly of collagen-mimetic mini-fibrils.Protein Sci. 2019 Sep;28(9):1640-1651. doi: 10.1002/pro.3679. Protein Sci. 2019. PMID: 31299125 Free PMC article.
-
Synthetic collagen mimics: self-assembly of homotrimers, heterotrimers and higher order structures.Chem Soc Rev. 2010 Sep;39(9):3510-27. doi: 10.1039/b919455j. Epub 2010 Jul 30. Chem Soc Rev. 2010. PMID: 20676409 Review.
-
Collagen Mimetic Peptides.Bioengineering (Basel). 2021 Jan 5;8(1):5. doi: 10.3390/bioengineering8010005. Bioengineering (Basel). 2021. PMID: 33466358 Free PMC article. Review.
Cited by
-
Exploring the Fibrous Nature of Single-Stranded DNA-Collagen Complexes: Nanostructural Observations and Physicochemical Insights.ACS Omega. 2024 Jul 11;9(29):32052-32058. doi: 10.1021/acsomega.4c04104. eCollection 2024 Jul 23. ACS Omega. 2024. PMID: 39072094 Free PMC article.
-
Crafting of functional biomaterials by directed molecular self-assembly of triple helical peptide building blocks.Interface Focus. 2017 Dec 6;7(6):20160138. doi: 10.1098/rsfs.2016.0138. Epub 2017 Oct 20. Interface Focus. 2017. PMID: 29147553 Free PMC article. Review.
-
Fluorinated Protein and Peptide Materials for Biomedical Applications.Pharmaceuticals (Basel). 2022 Sep 28;15(10):1201. doi: 10.3390/ph15101201. Pharmaceuticals (Basel). 2022. PMID: 36297312 Free PMC article. Review.
-
Collagen- and gelatine-based films sealing vascular prostheses: evaluation of the degree of crosslinking for optimal blood impermeability.J Mater Sci Mater Med. 2009 Oct;20(10):1979-89. doi: 10.1007/s10856-009-3778-1. Epub 2009 May 18. J Mater Sci Mater Med. 2009. PMID: 19449199
-
Hybrid multicomponent hydrogels for tissue engineering.Macromol Biosci. 2009 Feb 11;9(2):140-56. doi: 10.1002/mabi.200800284. Macromol Biosci. 2009. PMID: 19107720 Free PMC article. Review.
References
-
- Khoshnoodi J, Cartailler J-P, Alvares K, Veis A, Hudson BG. Molecular recognition in the assembly of collagens: Terminal noncollagenous domains are key recognition modules in the formation of triple helical protomers. J Biol Chem. 2006;281:38117–38121. - PubMed
-
- Carlier M-F, Pantaloni D. Control of actin assembly dynamics in cell motility. J Biol Chem. 2007;282:23005–23009. - PubMed
-
- Binder WH, Smrzka OW. Self-assembly of fibers and fibrils. Angew Chem Int Ed. 2006;45:7324–7328. - PubMed
-
- Laidman J, Forse GJ, Yeates TO. Conformational change and assembly through edge β strands in transthyretin and other amyloid proteins. Acc Chem Res. 2006;39:576–583. - PubMed
-
- Ricard-Blum S, Ruggerio F, van der Rest M. The collagen superfamily. Top Curr Chem. 2005;247:35–84.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

