Biologically active Phytophthora mating hormone prepared by catalytic asymmetric total synthesis
- PMID: 18559862
- PMCID: PMC2438403
- DOI: 10.1073/pnas.0709289105
Biologically active Phytophthora mating hormone prepared by catalytic asymmetric total synthesis
Abstract
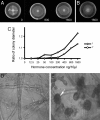
A Phytophthora mating hormone with an array of 1,5-stereogenic centers has been synthesized by using our recently developed methodology of catalytic enantioselective conjugate addition of Grignard reagents. We applied this methodology in a diastereo- and enantioselective iterative route and obtained two of the 16 possible stereoisomers of Phytophthora hormone alpha1. These synthetic stereoisomers induced the formation of sexual spores (oospores) in A2 mating type strains of three heterothallic Phytophthora species, P. infestans, P. capsici, and P. nicotianae but not in A1 mating type strains. The response was concentration-dependent, and the oospores were viable. These results demonstrate that the biological activity of the synthetic hormone resembles that of the natural hormone alpha1. Mating hormones are essential components in the sexual life cycle of a variety of organisms. For plant pathogens like Phytophthora, sexual reproduction is important as a source of genetic variation. Moreover, the thick-walled oospores are the most durable propagules that can survive harsh environmental conditions. Sexual reproduction can thus greatly affect disease epidemics. The availability of synthetic compounds mimicking the activity of Phytophthora mating hormone will be instrumental for further unravelling sexual reproduction in this important group of plant pathogens.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

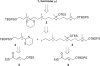

 CH-COSEt in CH2Cl2 at 40°C. (d) MeMgBr and 2 mol% (R, S)-7 in tBuOME at −78°C. (e) DIBALH in CH2Cl2 at −40°C. (f) TBDPSCl, Et3N, and DMAP in CH2Cl2 at room temperature. (g) AcOH, THF, and H2O (3/2/2) at 50°C. (h) IBX in EtOAc at 50°C. (i) HS(CH2)3SH and BF3·OEt2 in CH2 at −78°C.
CH-COSEt in CH2Cl2 at 40°C. (d) MeMgBr and 2 mol% (R, S)-7 in tBuOME at −78°C. (e) DIBALH in CH2Cl2 at −40°C. (f) TBDPSCl, Et3N, and DMAP in CH2Cl2 at room temperature. (g) AcOH, THF, and H2O (3/2/2) at 50°C. (h) IBX in EtOAc at 50°C. (i) HS(CH2)3SH and BF3·OEt2 in CH2 at −78°C.
 CH-COCH3 in THF at 66°C. (d) H2 and Pd/C in EtOAc at room temperature. (e) CH2
CH-COCH3 in THF at 66°C. (d) H2 and Pd/C in EtOAc at room temperature. (e) CH2 CH-CH
CH-CH CH
CH COTMS(OEt), TBAT, 10 mol% Cu(OTf)2, and 10 mol% (R)-Tol-BINAP in THF at room temperature. (f) H2, Pd/C in EtOAc at room temperature. (g) DIBALH in hexane at −78°C. (h) PPh3
COTMS(OEt), TBAT, 10 mol% Cu(OTf)2, and 10 mol% (R)-Tol-BINAP in THF at room temperature. (f) H2, Pd/C in EtOAc at room temperature. (g) DIBALH in hexane at −78°C. (h) PPh3 CH-COSEt in CH2Cl2 at 40°C. (i) TESOTf and 2,6-lutidine in CH2Cl2 at 0°C to room temperature. (j) MeMgBr and 2 mol% (S,R)-7 in BuOMe at −78°C. (k) DIBALH in CH2Cl2 at −40°C. (l) PPh3, I2, and imidazole in CH2Cl2 at room temperature.
CH-COSEt in CH2Cl2 at 40°C. (i) TESOTf and 2,6-lutidine in CH2Cl2 at 0°C to room temperature. (j) MeMgBr and 2 mol% (S,R)-7 in BuOMe at −78°C. (k) DIBALH in CH2Cl2 at −40°C. (l) PPh3, I2, and imidazole in CH2Cl2 at room temperature.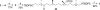

Similar articles
-
Synthesis and absolute configuration of hormone alpha1.Nat Chem Biol. 2008 Apr;4(4):235-7. doi: 10.1038/nchembio.74. Epub 2008 Feb 24. Nat Chem Biol. 2008. PMID: 18297064
-
Universality of the Phytophthora mating hormones and diversity of their production profile.Sci Rep. 2017 Jul 10;7(1):5007. doi: 10.1038/s41598-017-05380-3. Sci Rep. 2017. PMID: 28694506 Free PMC article.
-
Total synthesis of Phytophthora mating hormone α1.Org Lett. 2010 Nov 19;12(22):5166-9. doi: 10.1021/ol102177j. Epub 2010 Oct 25. Org Lett. 2010. PMID: 20973505
-
Synthesis of microbial signaling molecules and their stereochemistry-activity relationships.Biosci Biotechnol Biochem. 2011;75(8):1418-29. doi: 10.1271/bbb.110283. Epub 2011 Aug 7. Biosci Biotechnol Biochem. 2011. PMID: 21821958 Review.
-
The spores of Phytophthora: weapons of the plant destroyer.Nat Rev Microbiol. 2005 Jan;3(1):47-58. doi: 10.1038/nrmicro1064. Nat Rev Microbiol. 2005. PMID: 15608699 Review.
Cited by
-
Parallels in intercellular communication in oomycete and fungal pathogens of plants and humans.PLoS Pathog. 2012;8(12):e1003028. doi: 10.1371/journal.ppat.1003028. Epub 2012 Dec 13. PLoS Pathog. 2012. PMID: 23271965 Free PMC article. Review. No abstract available.
-
Comparative transcriptome analysis of R3a and Avr3a-mediated defense responses in transgenic tomato.PeerJ. 2021 Aug 9;9:e11965. doi: 10.7717/peerj.11965. eCollection 2021. PeerJ. 2021. PMID: 34434667 Free PMC article.
-
Sterol-Sensing Domain (SSD)-Containing Proteins in Sterol Auxotrophic Phytophthora capsici Mediate Sterol Signaling and Play a Role in Asexual Reproduction and Pathogenicity.Microbiol Spectr. 2023 Feb 14;11(1):e0379722. doi: 10.1128/spectrum.03797-22. Epub 2023 Jan 11. Microbiol Spectr. 2023. PMID: 36629430 Free PMC article.
-
Asymmetric synthesis and structure elucidation of a glycerophospholipid from Mycobacterium tuberculosis.J Lipid Res. 2010 May;51(5):1017-22. doi: 10.1194/jlr.M001982. Epub 2009 Nov 5. J Lipid Res. 2010. PMID: 19965610 Free PMC article.
-
Identification of the First Oomycete Mating-type Locus Sequence in the Grapevine Downy Mildew Pathogen, Plasmopara viticola.Curr Biol. 2020 Oct 19;30(20):3897-3907.e4. doi: 10.1016/j.cub.2020.07.057. Epub 2020 Aug 13. Curr Biol. 2020. PMID: 32795448 Free PMC article.
References
-
- Erwin DC, Ribeiro OK. Phytophthora Diseases Worldwide. St. Paul, MN: American Phytopathological Society; 1996.
-
- Fry WE, Goodwin SB. Resurgence of the Irish potato famine fungus. Bioscience. 1997;47:363–371.
-
- Govers F, Latijnhouwers M. In: Encyclopedia of Plant and Crop Science. Goodman RM, editor. New York: Marcel Dekker; 2004. pp. 1–5. - DOI
-
- McDonald BA, Linde C. Pathogen population genetics, evolutionary potential, and durable resistance. Annu Rev Phytopathol. 2002;40:349–379. - PubMed
-
- Qi J, et al. Characterization of a Phytophthora mating hormone. Science. 2005;309:1828. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

