Patients with acute myeloid leukemia and RAS mutations benefit most from postremission high-dose cytarabine: a Cancer and Leukemia Group B study
- PMID: 18559876
- PMCID: PMC2653132
- DOI: 10.1200/JCO.2007.14.0418
Patients with acute myeloid leukemia and RAS mutations benefit most from postremission high-dose cytarabine: a Cancer and Leukemia Group B study
Abstract
Purpose: RAS mutations occur in 12% to 27% of patients with acute myeloid leukemia (AML) and enhance sensitivity to cytarabine in vitro. We examined whether RAS mutations impact response to cytarabine in vivo.
Patients and methods: One hundred eighty-five patients with AML achieving complete remission on Cancer and Leukemia Group B study 8525 and randomly assigned to one of three doses of cytarabine postremission were screened for RAS mutations. We assessed the impact of cytarabine dose on cumulative incidence of relapse (CIR) of patients with (mutRAS) and without (wild-type; wtRAS) RAS mutations.
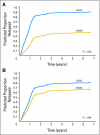
Results: Thirty-four patients (18%) had RAS mutations. With 12.9 years median follow-up, the 10-year CIR was similar for mutRAS and wtRAS patients (65% v 73%; P = .31). However, mutRAS patients receiving high-dose cytarabine consolidation (HDAC; 3 g/m(2) every 12 hours on days 1, 3, and 5 or 400 mg/m(2)/d x 5 days) had the lowest 10-year CIR, 45%, compared with 68% for wtRAS patients receiving HDAC and 80% and 100%, respectively, for wtRAS and mutRAS patients receiving low-dose cytarabine (LDAC; 100 mg/m(2)/d x 5 days; overall comparison, P < .001). Multivariable analysis revealed an interaction of cytarabine dose and RAS status (P = .06). After adjusting for this interaction and cytogenetics (core binding factor [CBF] AML v non-CBF AML), wtRAS patients receiving HDAC had lower relapse risk than wtRAS patients receiving LDAC (hazard ratio [HR] = 0.67; P = .04); however, mutRAS patients receiving HDAC had greater reduction in relapse risk (HR = 0.28; P = .002) compared with mutRAS patients treated with LDAC.
Conclusion: AML patients carrying mutRAS benefit from higher cytarabine doses more than wtRAS patients. This seems to be the first example of an activating oncogene mutation favorably modifying response to higher drug doses in AML.
Figures



Comment in
-
Molecular classification of acute myeloid leukemia: are we there yet?J Clin Oncol. 2008 Oct 1;26(28):4539-41. doi: 10.1200/JCO.2008.16.4293. Epub 2008 Jun 16. J Clin Oncol. 2008. PMID: 18559871 No abstract available.
References
-
- Bos JL: RAS oncogenes in human cancer: A review. Cancer Res 49:4682-4689, 1989. [Erratum: Cancer Res 50:1352, 1990] - PubMed
-
- Paquette RL, Landaw EM, Pierre RV, et al: N-RAS mutations are associated with poor prognosis and increased risk of leukemia in myelodysplastic syndrome. Blood 82:590-599, 1993 - PubMed
-
- Bos JL, Verlaan-de Vries M, van der Eb AJ, et al: Mutations in N-RAS predominate in acute myeloid leukemia. Blood 69:1237-1241, 1987 - PubMed
-
- Radich JP, Kopecky KJ, Willman CL, et al: N-RAS mutations in adult de novo acute myelogenous leukemia: Prevalence and clinical significance. Blood 76:801-807, 1990 - PubMed
-
- Coghlan DW, Morley AA, Matthews JP, et al: The incidence and prognostic significance of mutations in codon 13 of the N-RAS gene in acute myeloid leukemia. Leukemia 8:1682-1687, 1994 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

