Autosomal recessive hypophosphatasia manifesting in utero with long bone deformity but showing spontaneous postnatal improvement
- PMID: 18559907
- PMCID: PMC2567856
- DOI: 10.1210/jc.2008-0318
Autosomal recessive hypophosphatasia manifesting in utero with long bone deformity but showing spontaneous postnatal improvement
Abstract
Context: Hypophosphatasia (HPP) is a heritable metabolic disorder of the skeleton that includes variable expressivity conditioned by gene dosage effect and the variety of mutations in the tissue nonspecific alkaline phosphatase (TNSALP) gene. Patient age when skeletal problems first manifest generally predicts the clinical course, with perinatal HPP causing bone disease in utero with postnatal lethality.
Objective: Our objective was to identify TNSALP mutations and characterize the inheritance pattern of a family with clinically variable HPP with one child manifesting in utero with long bone deformity but showing spontaneous prenatal and postnatal improvement.
Design: TNSALP enzyme and substrate analysis and TNSALP mutation analysis were performed on all family members.
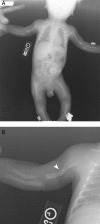
Patients: A boy with HPP showing long bone deformity that spontaneously improved in utero and after birth is described. His older brother has the childhood form of HPP without findings until after infancy. His parents and twin sister are clinically unaffected.
Results: Both boys are compound heterozygotes for the same missense mutations in TNSALP, documenting autosomal recessive inheritance for their HPP. The parents each carry one defective allele.
Conclusions: The patient is an autosomal recessive case of HPP with prenatal long bone deformity but with spontaneous prenatal and postnatal improvement. Thus, prenatal detection by sonography of bowing of long bones from HPP, even with autosomal recessive inheritance, does not necessarily predict lethality but can represent variable expressivity or the effects of modifiers on the TNSALP defect(s).
Figures



Similar articles
-
Hypophosphatasia: nonlethal disease despite skeletal presentation in utero (17 new cases and literature review).J Bone Miner Res. 2011 Oct;26(10):2389-98. doi: 10.1002/jbmr.454. J Bone Miner Res. 2011. PMID: 21713987 Review.
-
Hypophosphatasia: validation and expansion of the clinical nosology for children from 25 years experience with 173 pediatric patients.Bone. 2015 Jun;75:229-39. doi: 10.1016/j.bone.2015.02.022. Epub 2015 Feb 27. Bone. 2015. PMID: 25731960
-
Two novel mutations in the ALPL gene of unrelated Chinese children with Hypophosphatasia: case reports and literature review.BMC Pediatr. 2019 Nov 25;19(1):456. doi: 10.1186/s12887-019-1800-4. BMC Pediatr. 2019. PMID: 31760938 Free PMC article. Review.
-
Hypophosphatasia: Biochemical hallmarks validate the expanded pediatric clinical nosology.Bone. 2018 May;110:96-106. doi: 10.1016/j.bone.2018.01.022. Epub 2018 Jan 31. Bone. 2018. PMID: 29360619
-
Utility of genetic testing for prenatal presentations of hypophosphatasia.Mol Genet Metab. 2021 Mar;132(3):198-203. doi: 10.1016/j.ymgme.2021.01.009. Epub 2021 Jan 27. Mol Genet Metab. 2021. PMID: 33549410
Cited by
-
Effects of Infantile Hypophosphatasia on Human Dental Tissue.Calcif Tissue Int. 2023 Mar;112(3):308-319. doi: 10.1007/s00223-022-01041-4. Epub 2022 Nov 21. Calcif Tissue Int. 2023. PMID: 36414794 Free PMC article.
-
Genotype-Phenotype Associations in 72 Adults with Suspected ALPL-Associated Hypophosphatasia.Calcif Tissue Int. 2021 Mar;108(3):288-301. doi: 10.1007/s00223-020-00771-7. Epub 2020 Nov 15. Calcif Tissue Int. 2021. PMID: 33191482 Free PMC article.
-
Large-scale in vitro functional testing and novel variant scoring via protein modeling provide insights into alkaline phosphatase activity in hypophosphatasia.Hum Mutat. 2020 Jul;41(7):1250-1262. doi: 10.1002/humu.24010. Epub 2020 Mar 18. Hum Mutat. 2020. PMID: 32160374 Free PMC article.
-
Neonatal Bone Disorders.Front Pediatr. 2021 Apr 6;9:602552. doi: 10.3389/fped.2021.602552. eCollection 2021. Front Pediatr. 2021. PMID: 33889553 Free PMC article. Review.
-
Multigenerational case examples of hypophosphatasia: Challenges in genetic counseling and disease management.Mol Genet Metab Rep. 2020 Oct 21;25:100661. doi: 10.1016/j.ymgmr.2020.100661. eCollection 2020 Dec. Mol Genet Metab Rep. 2020. PMID: 33101980 Free PMC article.
References
-
- Smith M, Weiss MJ, Griffin CA, Murray JC, Buetow KH, Emanuel BS, Henthorn PS, Harris H 1988 Regional assignment of the gene for human liver/bone/kidney alkaline phosphatase to chromosome 1p36.1-p34. Genomics 2:139–143 - PubMed
-
- Whyte MP 1994 Hypophosphatasia and the role of alkaline phosphatase in skeletal mineralization. Endocr Rev 15:439–461 - PubMed
-
- Whyte MP 2001 Hypophosphatasia. In: Scriver CR, Beaudet AL, Sly WS, Valle D, eds. The metabolic and molecular bases of inherited disease. 8th ed. New York: McGraw-Hill; 5313–5329
-
- McCance RA, Morrison AB, Dent CE 1955 The excretion of phosphoethanolamine and hypophosphatasia. Lancet 268:131 - PubMed

