Neurooncology clinical trial design for targeted therapies: lessons learned from the North American Brain Tumor Consortium
- PMID: 18559968
- PMCID: PMC2666238
- DOI: 10.1215/15228517-2008-021
Neurooncology clinical trial design for targeted therapies: lessons learned from the North American Brain Tumor Consortium
Abstract
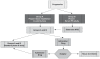
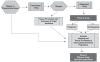
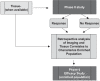
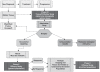
The North American Brain Tumor Consortium (NABTC) is a multi-institutional consortium with the primary objective of evaluating novel therapeutic strategies through early phase clinical trials. The NABTC has made substantial changes to the design and methodology of its trials since its inception in 1994. These changes reflect developments in technology, new types of therapies, and advances in our understanding of tumor biology and biological markers. We identify the challenges of early clinical assessment of therapeutic agents by reviewing the clinical trial effort of the NABTC and the evolution of the protocol template used to design trials. To better prioritize effort and allocation of patient resources and funding, we propose an integrated clinical trial design for the early assessment of efficacy of targeted therapies in neurooncology. This design would mandate tissue acquisition prior to therapeutic intervention with the drug, allowing prospective evaluation of its effects. It would also include a combined phase 0/I pharmacokinetic study to determine the safety and biologically optimal dose of the agent and to verify successful modulation of the target prior to initiating a larger, phase II efficacy study.
Figures



References
-
- Chang SM, Wen P, Cloughesy T, et al. Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Invest New Drugs. 2005;23:357–361. - PubMed
-
- Cloughesy TF, Wen PY, Robins HI, et al. Phase II trial of tipifarnib in patients with recurrent malignant glioma either receiving or not receiving enzyme-inducing antiepileptic drugs: a North American Brain Tumor Consortium Study. J Clin Oncol. 2006;24:3651–3656. - PubMed
-
- Wen PY, Yung WK, Lamborn KR, et al. Phase I/II study of imatinib mesylate for recurrent malignant gliomas: North American Brain Tumor Consortium Study 99-08. Clin Cancer Res. 2006;12:4899–4907. - PubMed
-
- Fox E, Curt GA, Balis FM. Clinical trial design for target-based therapy. Oncologist. 2002;7:401–409. - PubMed
-
- Ma BB, Britten CD, Siu LL. Clinical trial designs for targeted agents. Hematol Oncol Clin North Am. 2002;16:1287–1305. - PubMed
Publication types
MeSH terms
Grants and funding
- CA 62426/CA/NCI NIH HHS/United States
- CA 62422/CA/NCI NIH HHS/United States
- UL1 TR000005/TR/NCATS NIH HHS/United States
- M01-RR0865/RR/NCRR NIH HHS/United States
- M01-RR00079/RR/NCRR NIH HHS/United States
- U01 CA062399/CA/NCI NIH HHS/United States
- M01-RR00056/RR/NCRR NIH HHS/United States
- CA 62421/CA/NCI NIH HHS/United States
- CA 62405/CA/NCI NIH HHS/United States
- CA 62407-13/CA/NCI NIH HHS/United States
- 1U11RR025011/RR/NCRR NIH HHS/United States
- CA 105663-03/CA/NCI NIH HHS/United States
- CA 62412/CA/NCI NIH HHS/United States
- CA 16672/CA/NCI NIH HHS/United States
- CA 62399/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

