Id1 is a common downstream target of oncogenic tyrosine kinases in leukemic cells
- PMID: 18559972
- PMCID: PMC2518899
- DOI: 10.1182/blood-2007-07-103010
Id1 is a common downstream target of oncogenic tyrosine kinases in leukemic cells
Abstract
Oncogenic tyrosine kinases, such as BCR-ABL, TEL-ABL, TEL-PDGFbetaR, and FLT3-ITD, play a major role in the development of hematopoietic malignancy. They activate many of the same signal transduction pathways. To identify the critical target genes required for transformation in hematopoietic cells, we used a comparative gene expression strategy in which selective small molecules were applied to 32Dcl3 cells that had been transformed to factor-independent growth by these respective oncogenic alleles. We identified inhibitor of DNA binding 1 (Id1), a gene involved in development, cell cycle, and tumorigenesis, as a common target of these oncogenic kinases. These findings were prospectively confirmed in cell lines and primary bone marrow cells engineered to express the respective tyrosine kinase alleles and were also confirmed in vivo in murine models of disease. Moreover, human AML cell lines Molm-14 and K562, which express the FLT3-ITD and BCR-ABL tyrosine kinases, respectively, showed high levels of Id1 expression. Antisense and siRNA based knockdown of Id1-inhibited growth of these cells associated with increased p27(Kip1) expression and increased sensitivity to Trail-induced apoptosis. These findings indicate that Id1 is an important target of constitutively activated tyrosine kinases and may be a therapeutic target for leukemias associated with oncogenic tyrosine kinases.
Figures
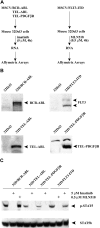

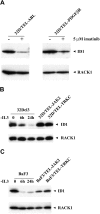
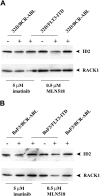
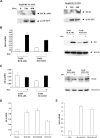

References
-
- Daley GQ, Van Etten RA, Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990;247:824–830. - PubMed
-
- Van Etten RA, Jackson P, Baltimore D. The mouse type IV c-abl gene product is a nuclear protein, and activation of transforming ability is associated with cytoplasmic localization. Cell. 1989;58:669–678. - PubMed
-
- Okuda K, Golub TR, Gilliland DG, Griffin JD. p210BCR/ABL, p190BCR/ABL, and TEL/ABL activate similar signal transduction pathways in hematopoietic cell lines. Oncogene. 1996;13:1147–1152. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

