Overexpression of delta-like 4 induces arterialization and attenuates vessel formation in developing mouse embryos
- PMID: 18559979
- PMCID: PMC2518882
- DOI: 10.1182/blood-2007-09-112748
Overexpression of delta-like 4 induces arterialization and attenuates vessel formation in developing mouse embryos
Abstract
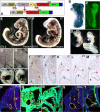
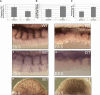
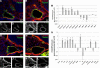
The importance of Notch signaling pathway in the regulation of vascular development and angiogenesis is suggested by the expression of Notch receptors and ligands in vascular endothelial cells (ECs) and the observed vascular phenotypes in mutants of Notch receptors or ligands, especially Dll4. DLL4 is specifically expressed in arterial ECs during development, and haplo-insufficiency is embryonically lethal in mice. To address the role of Dll4 in vascular development, we produced mDll4 conditionally overexpressed transgenic mice that were crossed with constitutive recombinase cre lines. Double transgenic embryos displayed grossly enlarged dorsal aortae (DA) and died before embryonic day 10.5 (E10.5), showing a variable degree of premature arteriovenous fusion. Veins displayed ectopic expression of arterial markers. Other defects included reduced vascular sprouting, EC proliferation, and migration. mDll4 overexpression also inhibited VEGF signaling and increased fibronectin accumulation around the vessels. In vitro and in vivo studies of DLL4-FL (Dll4-full-length) in ECs recapitulate many of the mDll4 transgenics findings, including decreased tube formation, reduced vascular branching, fewer vessels, increased pericyte recruitment, and increased fibronectin expression. These results establish the role of Dll4 in arterial identity determination, and regulation of angiogenesis subject to dose and location.
Figures



References
-
- Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. - PubMed
-
- Gerety SS, Wang HU, Chen ZF, Anderson DJ. Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Mol Cell. 1999;4:403–414. - PubMed
-
- Gale NW, Baluk P, Pan L, et al. Ephrin-B2 selectively marks arterial vessels and neovascularization sites in the adult, with expression in both endothelial and smooth-muscle cells. Dev Biol. 2001;230:151–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

