Direct evidence of iNOS-mediated in vivo free radical production and protein oxidation in acetone-induced ketosis
- PMID: 18559982
- PMCID: PMC2519762
- DOI: 10.1152/ajpendo.00015.2008
Direct evidence of iNOS-mediated in vivo free radical production and protein oxidation in acetone-induced ketosis
Abstract
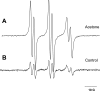
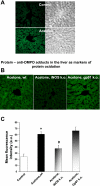
Diabetic patients frequently encounter ketosis that is characterized by the breakdown of lipids with the consequent accumulation of ketone bodies. Several studies have demonstrated that reactive species are likely to induce tissue damage in diabetes, but the role of the ketone bodies in the process has not been fully investigated. In this study, electron paramagnetic resonance (EPR) spectroscopy combined with novel spin-trapping and immunological techniques has been used to investigate in vivo free radical formation in a murine model of acetone-induced ketosis. A six-line EPR spectrum consistent with the alpha-(4-pyridyl-1-oxide)-N-t-butylnitrone radical adduct of a carbon-centered lipid-derived radical was detected in the liver extracts. To investigate the possible enzymatic source of these radicals, inducible nitric oxide synthase (iNOS) and NADPH oxidase knockout mice were used. Free radical production was unchanged in the NADPH oxidase knockout but much decreased in the iNOS knockout mice, suggesting a role for iNOS in free radical production. Longer-term exposure to acetone revealed iNOS overexpression in the liver together with protein radical formation, which was detected by confocal microscopy and a novel immunospin-trapping method. Immunohistochemical analysis revealed enhanced lipid peroxidation and protein oxidation as a consequence of persistent free radical generation after 21 days of acetone treatment in control and NADPH oxidase knockout but not in iNOS knockout mice. Taken together, our data demonstrate that acetone administration, a model of ketosis, can lead to protein oxidation and lipid peroxidation through a free radical-dependent mechanism driven mainly by iNOS overexpression.
Figures



Similar articles
-
Free radical production requires both inducible nitric oxide synthase and xanthine oxidase in LPS-treated skin.Proc Natl Acad Sci U S A. 2006 Mar 21;103(12):4616-21. doi: 10.1073/pnas.0510352103. Epub 2006 Mar 14. Proc Natl Acad Sci U S A. 2006. PMID: 16537416 Free PMC article.
-
In vivo lipid-derived free radical formation by NADPH oxidase in acute lung injury induced by lipopolysaccharide: a model for ARDS.FASEB J. 2002 Nov;16(13):1713-20. doi: 10.1096/fj.02-0331com. FASEB J. 2002. PMID: 12409313
-
In vivo evidence of free radical generation in the mouse lung after exposure to Pseudomonas aeruginosa bacterium: an ESR spin-trapping investigation.Free Radic Res. 2012 May;46(5):645-55. doi: 10.3109/10715762.2012.667089. Epub 2012 Mar 16. Free Radic Res. 2012. PMID: 22339444 Free PMC article.
-
Nitric oxide trapping of the tyrosyl radical-chemistry and biochemistry.Toxicology. 2002 Aug 1;177(1):1-9. doi: 10.1016/s0300-483x(02)00191-9. Toxicology. 2002. PMID: 12126791 Review.
-
Nitric Oxide-elicited Resistance to Antitumor Photodynamic Therapy via Inhibition of Membrane Free Radical-mediated Lipid Peroxidation.Photochem Photobiol. 2021 Jul;97(4):653-663. doi: 10.1111/php.13373. Epub 2021 Jan 19. Photochem Photobiol. 2021. PMID: 33369741 Review.
Cited by
-
Immature myeloid cells induced by a high-fat diet contribute to liver inflammation.Hepatology. 2009 Nov;50(5):1412-20. doi: 10.1002/hep.23148. Hepatology. 2009. PMID: 19708080 Free PMC article.
-
Oxidative stress induces protein and DNA radical formation in follicular dendritic cells of the germinal center and modulates its cell death patterns in late sepsis.Free Radic Biol Med. 2011 Apr 15;50(8):988-99. doi: 10.1016/j.freeradbiomed.2010.12.037. Epub 2011 Jan 4. Free Radic Biol Med. 2011. PMID: 21215311 Free PMC article.
-
In Vivo Immuno-Spin Trapping: Imaging the Footprints of Oxidative Stress.Curr Protoc Cytom. 2015 Oct 1;74:12.42.1-12.42.11. doi: 10.1002/0471142956.cy1242s74. Curr Protoc Cytom. 2015. PMID: 26423693 Free PMC article.
-
Immuno-spin trapping of a post-translational carboxypeptidase B1 radical formed by a dual role of xanthine oxidase and endothelial nitric oxide synthase in acute septic mice.Free Radic Biol Med. 2009 Feb 15;46(4):454-61. doi: 10.1016/j.freeradbiomed.2008.10.046. Epub 2008 Nov 7. Free Radic Biol Med. 2009. PMID: 19049863 Free PMC article.
-
Transcriptomic analyses and leukocyte telomere length measurement in subjects exposed to severe recent stressful life events.Transl Psychiatry. 2017 Feb 21;7(2):e1042. doi: 10.1038/tp.2017.5. Transl Psychiatry. 2017. PMID: 28221367 Free PMC article.
References
-
- Adrogue HJ, Wilson H, Boyd AE 3rd, Suki WN, Eknoyan G. Plasma acid-base patterns in diabetic ketoacidosis. N Engl J Med 307: 1603–1610, 1982. - PubMed
-
- Casazza JP, Felver ME, Veech RL. The metabolism of acetone in rat. J Biol Chem 259: 231–236, 1984. - PubMed
-
- Detweiler CD, Deterding LJ, Tomer KB, Chignell CF, Germolec D, Mason RP. Immunological identification of the heart myoglobin radical formed by hydrogen peroxide. Free Radic Biol Med 33: 364–369, 2002. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

