Modulation of P-glycoprotein at the blood-brain barrier: opportunities to improve central nervous system pharmacotherapy
- PMID: 18560012
- PMCID: PMC2634288
- DOI: 10.1124/pr.107.07109
Modulation of P-glycoprotein at the blood-brain barrier: opportunities to improve central nervous system pharmacotherapy
Abstract
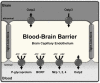
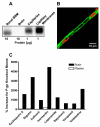
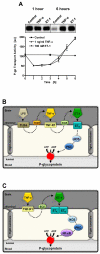
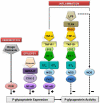
Pharmacotherapy of central nervous system (CNS) disorders (e.g., neurodegenerative diseases, epilepsy, brain cancer, and neuro-AIDS) is limited by the blood-brain barrier. P-glycoprotein, an ATP-driven, drug efflux transporter, is a critical element of that barrier. High level of expression, luminal membrane location, multispecificity, and high transport potency make P-glycoprotein a selective gatekeeper of the blood-brain barrier and thus a primary obstacle to drug delivery into the brain. As such, P-glycoprotein limits entry into the CNS for a large number of prescribed drugs, contributes to the poor success rate of CNS drug candidates, and probably contributes to patient-to-patient variability in response to CNS pharmacotherapy. Modulating P-glycoprotein could therefore improve drug delivery into the brain. Here we review the current understanding of signaling mechanisms responsible for the modulation of P-glycoprotein activity/expression at the blood-brain barrier with an emphasis on recent studies from our laboratories. Using intact brain capillaries from rats and mice, we have identified multiple extracellular and intracellular signals that regulate this transporter; several signaling pathways have been mapped. Three pathways are triggered by elements of the brain's innate immune response, one by glutamate, one by xenobiotic-nuclear receptor (pregnane X receptor) interactions, and one by elevated beta-amyloid levels. Signaling is complex, with several pathways sharing common signaling elements [tumor necrosis factor (TNF) receptor 1, endothelin (ET) B receptor, protein kinase C, and nitric-oxide synthase), suggesting a regulatory network. Several pathways include autocrine/paracrine elements, involving release of the proinflammatory cytokine, TNF-alpha, and the polypeptide hormone, ET-1. Finally, several steps in signaling are potential therapeutic targets that could be used to modulate P-glycoprotein activity in the clinic.
Figures
Similar articles
-
Tumor necrosis factor alpha and endothelin-1 increase P-glycoprotein expression and transport activity at the blood-brain barrier.Mol Pharmacol. 2007 Mar;71(3):667-75. doi: 10.1124/mol.106.029512. Epub 2006 Nov 28. Mol Pharmacol. 2007. PMID: 17132686
-
Rapid modulation of P-glycoprotein-mediated transport at the blood-brain barrier by tumor necrosis factor-alpha and lipopolysaccharide.Mol Pharmacol. 2006 Feb;69(2):462-70. doi: 10.1124/mol.105.017954. Epub 2005 Nov 8. Mol Pharmacol. 2006. PMID: 16278373
-
Activation of PKC isoform beta(I) at the blood-brain barrier rapidly decreases P-glycoprotein activity and enhances drug delivery to the brain.J Cereb Blood Flow Metab. 2010 Jul;30(7):1373-83. doi: 10.1038/jcbfm.2010.21. Epub 2010 Mar 3. J Cereb Blood Flow Metab. 2010. PMID: 20197783 Free PMC article.
-
Drug transport to the brain: key roles for the efflux pump P-glycoprotein in the blood-brain barrier.Vascul Pharmacol. 2002 Jun;38(6):339-48. doi: 10.1016/s1537-1891(02)00201-x. Vascul Pharmacol. 2002. PMID: 12529928 Review.
-
Modulation of p-glycoprotein transport function at the blood-brain barrier.Exp Biol Med (Maywood). 2005 Feb;230(2):118-27. doi: 10.1177/153537020523000206. Exp Biol Med (Maywood). 2005. PMID: 15673560 Review.
Cited by
-
Physiology and pathophysiology of the blood-brain barrier: P-glycoprotein and occludin trafficking as therapeutic targets to optimize central nervous system drug delivery.J Investig Med. 2012 Dec;60(8):1131-40. doi: 10.2310/JIM.0b013e318276de79. J Investig Med. 2012. PMID: 23138008 Free PMC article. Review.
-
Regulation of P-Glycoprotein in the Brain.Int J Mol Sci. 2022 Nov 24;23(23):14667. doi: 10.3390/ijms232314667. Int J Mol Sci. 2022. PMID: 36498995 Free PMC article. Review.
-
Syntheses and in vitro biological evaluation of S1PR1 ligands and PET studies of four F-18 labeled radiotracers in the brain of nonhuman primates.Org Biomol Chem. 2018 Dec 5;16(47):9171-9184. doi: 10.1039/c8ob02609b. Org Biomol Chem. 2018. PMID: 30462126 Free PMC article.
-
HIV-1 Tat and opioids act independently to limit antiretroviral brain concentrations and reduce blood-brain barrier integrity.J Neurovirol. 2019 Aug;25(4):560-577. doi: 10.1007/s13365-019-00757-8. Epub 2019 May 17. J Neurovirol. 2019. PMID: 31102185 Free PMC article.
-
Overcoming the Blood-Brain Barrier: Successes and Challenges in Developing Nanoparticle-Mediated Drug Delivery Systems for the Treatment of Brain Tumours.Int J Nanomedicine. 2020 Apr 30;15:2999-3022. doi: 10.2147/IJN.S231479. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32431498 Free PMC article. Review.
References
-
- Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007a;100:158–173. - PubMed
-
- Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007b;100:174–190. - PubMed
-
- Anderson GD, Shen DD. Where is the evidence that p-glycoprotein limits brain uptake of antiepileptic drug and contributes to drug resistance in epilepsy? Epilepsia. 2007;48:2372–2374. - PubMed
-
- Babakhanian K, Bendayan M, Bendayan R. Localization of P-glycoprotein at the nuclear envelope of rat brain cells. Biochem Biophys Res Commun. 2007;361:301–306. - PubMed
-
- Baker EK, El-Osta A. The rise of DNA methylation and the importance of chromatin on multidrug resistance in cancer. Exp Cell Res. 2003;290:177–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

