Control of endothelial targeting and intracellular delivery of therapeutic enzymes by modulating the size and shape of ICAM-1-targeted carriers
- PMID: 18560419
- PMCID: PMC2810502
- DOI: 10.1038/mt.2008.127
Control of endothelial targeting and intracellular delivery of therapeutic enzymes by modulating the size and shape of ICAM-1-targeted carriers
Abstract
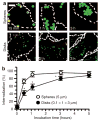
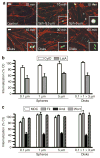
Endocytosis in endothelial cells (ECs) is important for many biomedical applications, including drug delivery by nano- and microscale carriers. However, little is known about how carrier geometry influences endothelial drug targeting, intracellular trafficking, and effects. We studied this using prototype polymer carriers of various sizes (0.1-10 mum) and shapes (spheres versus elliptical disks). Carriers were targeted to intercellular adhesion molecule 1 (ICAM-1), a transmembrane glycoprotein that is upregulated in many pathologies and used as a target for intraendothelial drug delivery. ECs internalized anti-ICAM-coated carriers of up to several microns in size via cell adhesion molecule-mediated endocytosis. This pathway is distinct from caveolar and clathrin endocytosis that operate for submicron-size objects. Carrier geometry was found to influence endothelial targeting in the vasculature, and the rate of endocytosis and lysosomal transport within ECs. Disks had longer half-lives in circulation and higher targeting specificity in mice, whereas spheres were endocytosed more rapidly. Micron-size carriers had prolonged residency in prelysosomal compartments, beneficial for endothelial antioxidant protection by delivered catalase. Submicron carriers trafficked to lysosomes more readily, optimizing effects of acid sphingomyelinase (ASM) enzyme replacement in a model of lysosomal storage disease. Therefore, rational design of carrier geometry will help optimize endothelium-targeted therapeutics.
Figures






References
-
- Song BW, Vinters HV, Wu D, Pardridge WM. Enhanced neuroprotective effects of basic fibroblast growth factor in regional brain ischemia after conjugation to a blood-brain barrier delivery vector. J Pharmacol Exp Ther. 2002;301:605–610. - PubMed
-
- Muzykantov V. Targeting drugs to pulmonary endothelium. Expert Opinion Drug Delivery. 2005;2:909–926. - PubMed
-
- Oh P, Li Y, Yu J, Durr E, Krasinska KM, Carver LA, et al. Subtractive proteomic mapping of the endothelial surface in lung and solid tumours for tissue-specific therapy. Nature. 2004;429:629–635. - PubMed
-
- Wilson A, He F, Li J, Ma Z, Pitt B, Li S. Targeted delivery of therapeutic oligonucleotides to pulmonary circulation. Adv Genet. 2005;54:21–41. - PubMed
-
- Molema G. Tumor vasculature directed drug targeting: applying new technologies and knowledge to the development of clinically relevant therapies. Pharm Res. 2002;19:1251–1258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

