Ammonium-acetate is sensed by gustatory and olfactory neurons in Caenorhabditis elegans
- PMID: 18560547
- PMCID: PMC2413426
- DOI: 10.1371/journal.pone.0002467
Ammonium-acetate is sensed by gustatory and olfactory neurons in Caenorhabditis elegans
Abstract
Background: Caenorhabditis elegans chemosensation has been successfully studied using behavioral assays that treat detection of volatile and water soluble chemicals as separate senses, analogous to smell and taste. However, considerable ambiguity has been associated with the attractive properties of the compound ammonium-acetate (NH(4)Ac). NH(4)Ac has been used in behavioral assays both as a chemosensory neutral compound and as an attractant.
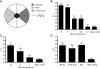
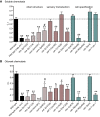
Methodology/main findings: Here we show that over a range of concentrations NH(4)Ac can be detected both as a water soluble attractant and as an odorant, and that ammonia and acetic acid individually act as olfactory attractants. We use genetic analysis to show that NaCl and NH(4)Ac sensation are mediated by separate pathways and that ammonium sensation depends on the cyclic nucleotide gated ion channel TAX-2/TAX-4, but acetate sensation does not. Furthermore we show that sodium-acetate (NaAc) and ammonium-chloride (NH(4)Cl) are not detected as Na(+) and Cl(-) specific stimuli, respectively.
Conclusions/significance: These findings clarify the behavioral response of C. elegans to NH(4)Ac. The results should have an impact on the design and interpretation of chemosensory experiments studying detection and adaptation to soluble compounds in the nematode Caenorhabditis elegans.
Conflict of interest statement
Figures

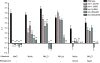
References
-
- Bargmann CI, Horvitz HR. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron. 1991;7:729–742. - PubMed
-
- Bargmann CI, Hartwieg E, Horvitz HR. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell. 1993;74:515–527. - PubMed
-
- Dusenbery DB. Analysis of chemotaxis in the nematode Caenorhabditis elegans by countercurrent separation. J Exp Zool. 1974;188:41–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

