Sarcomere alignment is regulated by myocyte shape
- PMID: 18561184
- PMCID: PMC4492320
- DOI: 10.1002/cm.20290
Sarcomere alignment is regulated by myocyte shape
Abstract
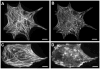
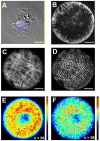
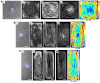
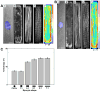
Cardiac organogenesis and pathogenesis are both characterized by changes in myocyte shape, cytoskeletal architecture, and the extracellular matrix (ECM). However, the mechanisms by which the ECM influences myocyte shape and myofibrillar patterning are unknown. We hypothesized that geometric cues in the ECM align sarcomeres by directing the actin network orientation. To test our hypothesis, we cultured neonatal rat ventricular myocytes on islands of micro-patterned ECM to measure how they remodeled their cytoskeleton in response to extracellular cues. Myocytes spread and assumed the shape of circular and rectangular islands and reorganized their cytoskeletons and myofibrillar arrays with respect to the ECM boundary conditions. Circular myocytes did not assemble predictable actin networks nor organized sarcomere arrays. In contrast, myocytes cultured on rectangular ECM patterns with aspect ratios ranging from 1:1 to 7:1 aligned their sarcomeres in predictable and repeatable patterns based on highly localized focal adhesion complexes. Examination of averaged alpha-actinin images revealed invariant sarcomeric registration irrespective of myocyte aspect ratio. Since the sarcomere sub-units possess a fixed length, this observation indicates that cytoskeleton configuration is length-limited by the extracellular boundary conditions. These results indicate that modification of the extracellular microenvironment induces dynamic reconfiguring of the myocyte shape and intracellular architecture. Furthermore, geometric boundaries such as corners induce localized myofibrillar anisotropy that becomes global as the myocyte aspect ratio increases.
(c) 2008 Wiley-Liss, Inc.
Figures
Similar articles
-
Control of myocyte remodeling in vitro with engineered substrates.In Vitro Cell Dev Biol Anim. 2009 Jul-Aug;45(7):343-50. doi: 10.1007/s11626-009-9182-9. Epub 2009 Feb 28. In Vitro Cell Dev Biol Anim. 2009. PMID: 19252956
-
Myofibrillar architecture in engineered cardiac myocytes.Circ Res. 2008 Aug 15;103(4):340-2. doi: 10.1161/CIRCRESAHA.108.182469. Epub 2008 Jul 17. Circ Res. 2008. PMID: 18635822 Free PMC article.
-
Self-organization of muscle cell structure and function.PLoS Comput Biol. 2011 Feb;7(2):e1001088. doi: 10.1371/journal.pcbi.1001088. Epub 2011 Feb 24. PLoS Comput Biol. 2011. PMID: 21390276 Free PMC article.
-
Intercellular and extracellular mechanotransduction in cardiac myocytes.Pflugers Arch. 2011 Jul;462(1):75-87. doi: 10.1007/s00424-011-0954-1. Epub 2011 Mar 25. Pflugers Arch. 2011. PMID: 21437600 Review.
-
Metabolic consequences of functional complexes of mitochondria, myofibrils and sarcoplasmic reticulum in muscle cells.J Exp Biol. 2003 Jun;206(Pt 12):2059-72. doi: 10.1242/jeb.00242. J Exp Biol. 2003. PMID: 12756288 Review.
Cited by
-
Aligned human cardiac syncytium for in vitro analysis of electrical, structural, and mechanical readouts.Biotechnol Bioeng. 2021 Jan;118(1):442-452. doi: 10.1002/bit.27582. Epub 2020 Oct 13. Biotechnol Bioeng. 2021. PMID: 32990953 Free PMC article.
-
Silencing of MYH7 ameliorates disease phenotypes in human iPSC-cardiomyocytes.Physiol Genomics. 2020 Jul 1;52(7):293-303. doi: 10.1152/physiolgenomics.00021.2020. Epub 2020 Jun 22. Physiol Genomics. 2020. PMID: 32567507 Free PMC article.
-
Effect of morphological change on the maturation of human induced pluripotent stem cell-derived cardiac tissue in rotating flow culture.Regen Ther. 2023 Sep 22;24:479-488. doi: 10.1016/j.reth.2023.09.002. eCollection 2023 Dec. Regen Ther. 2023. PMID: 37767182 Free PMC article.
-
Fibre-infused gel scaffolds guide cardiomyocyte alignment in 3D-printed ventricles.Nat Mater. 2023 Aug;22(8):1039-1046. doi: 10.1038/s41563-023-01611-3. Epub 2023 Jul 27. Nat Mater. 2023. PMID: 37500957 Free PMC article.
-
High content analysis identifies unique morphological features of reprogrammed cardiomyocytes.Sci Rep. 2018 Jan 19;8(1):1258. doi: 10.1038/s41598-018-19539-z. Sci Rep. 2018. PMID: 29352247 Free PMC article.
References
-
- Anversa P, Levicky V, Beghi C, McDonald SL, Kikkawa Y. Morphometry of exercise-induced right ventricular hypertrophy in the rat. Circ Res. 1983;52:57–64. - PubMed
-
- Anversa P, Ricci R, Olivetti G. Quantitative structural analysis of the myocardium during physiologic growth and induced cardiac hypertrophy: A review. J Amer Coll Cardiol. 1986;7:1140–1149. - PubMed
-
- Atherton BT, Meyer DM, Simpson DG. Assembly and remodelling of myofibrils and intercalated discs in cultured neonatal rat heart cells. J Cell Sci. 1986;86:233–248. - PubMed
-
- Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, Geiger B. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nature Cell Biol. 2001;3:466–472. - PubMed
-
- Beauchamp P, Yamada KA, Baertschi AJ, Green K, Kanter EM, Saffitz JE, Kléber AG. Relative contributions of connexins 40 and 43 to atrial impulse propagation in synthetic strands of neonatal and fetal murine cardiomyocytes. Circ Res. 2006;99:1216–1224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

