Effect of ramipril on renal function in patients with intermittent claudication
- PMID: 18561523
- PMCID: PMC2496985
- DOI: 10.2147/vhrm.s2340
Effect of ramipril on renal function in patients with intermittent claudication
Abstract
Background: The Heart Outcomes Prevention Study (HOPE) demonstrated that ramipril resulted in a blood-pressure-independent 25% reduction in cardiovascular events in patients with peripheral arterial disease (PAD). Despite this, general practitioners and vascular surgeons remain reluctant to prescribe ACE inhibitors in this group of patients because of concerns about renal artery stenosis (RAS). We aimed to define the effect of ramipril on renal function in patients with intermittent claudication (IC).
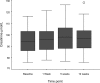
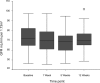
Methods and results: Of 132 unselected patients with IC entering the study 78 (59%) were excluded due to: current ACE inhibitor use (38%), renal impairment (serum creatinine above normal range) (15%), known severe RAS (1%) or unwillingness to participate (5%). The remaining 54 patients were titrated to 10 mg ramipril and renal function was monitored at 1, 5, and 12 weeks. Treatment was discontinued during titration in 5 patients due to symptoms (3) or lack of compliance (2). In the remainder, median [IQR] serum creatinine increased (94 [85.8-103.3] to 98 [88.0-106.5] micromol/L, p < or = 0.001) and median [IQR] GFR decreased (71.5 [64.6-82.3] to 68.7 [59.8-74.7] mL/min per 1.73 m2, p < or = 0.001) between baseline and 5 weeks. These changes were not considered clinically significant. By 12 weeks these values had returned almost to baseline (Cr 95.5 [88.0-103.25] micromol/L, GFR 71.8 [65.3-77.4] mL/min). No patient had a serum creatinine rise > 30%.
Conclusion: Most of patients with IC and a normal serum creatinine can be safely commenced on ramipril provided they are screened, titrated and monitored as described above. Studies in patients with borderline renal impairment (serum creatinine up to 30% above baseline) are on-going.
Figures
References
-
- Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine: Is this a cause for concern? Arch Int Med. 2000;160:685–93. - PubMed
-
- Bjorholt I, Andersson FL, Kahan T, et al. The cost-effectiveness of ramipril in the treatment of patients at high risk of cardiovascular events: a Swedish sub-study to the HOPE study. J Int Med. 2002;251:508–17. - PubMed
-
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

