Transurethral ultrasound applicators with dynamic multi-sector control for prostate thermal therapy: in vivo evaluation under MR guidance
- PMID: 18561684
- PMCID: PMC2673638
- DOI: 10.1118/1.2900131
Transurethral ultrasound applicators with dynamic multi-sector control for prostate thermal therapy: in vivo evaluation under MR guidance
Abstract
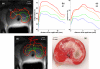
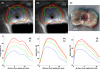
The purpose of this study was to explore the feasibility and performance of a multi-sectored tubular array transurethral ultrasound applicator for prostate thermal therapy, with potential to provide dynamic angular and length control of heating under MR guidance without mechanical movement of the applicator. Test configurations were fabricated, incorporating a linear array of two multi-sectored tubular transducers (7.8-8.4 MHz, 3 mm OD, 6 mm length), with three 120 degrees independent active sectors per tube. A flexible delivery catheter facilitated water cooling (100 ml min(-1)) within an expandable urethral balloon (35 mm long x 10 mm diameter). An integrated positioning hub allows for rotating and translating the transducer assembly within the urethral balloon for final targeting prior to therapy delivery. Rotational beam plots indicate approximately 90 degrees-100 degrees acoustic output patterns from each 120 degrees transducer sector, negligible coupling between sectors, and acoustic efficiencies between 41% and 53%. Experiments were performed within in vivo canine prostate (n = 3), with real-time MR temperature monitoring in either the axial or coronal planes to facilitate control of the heating profiles and provide thermal dosimetry for performance assessment. Gross inspection of serial sections of treated prostate, exposed to TTC (triphenyl tetrazolium chloride) tissue viability stain, allowed for direct assessment of the extent of thermal coagulation. These devices created large contiguous thermal lesions (defined by 52 degrees C maximum temperature, t43 = 240 min thermal dose contours, and TTC tissue sections) that extended radially from the applicator toward the border of the prostate (approximately15 mm) during a short power application (approximately 8-16 W per active sector, 8-15 min), with approximately 200 degrees or 360 degrees sector coagulation demonstrated depending upon the activation scheme. Analysis of transient temperature profiles indicated progression of lethal temperature and thermal dose contours initially centered on each sector that coalesced within approximately 5 min to produce uniform and contiguous zones of thermal destruction between sectors, with smooth outer boundaries and continued radial propagation in time. The dimension of the coagulation zone along the applicator was well-defined by positioning and active array length. Although not as precise as rotating planar and curvilinear devices currently under development for MR-guided procedures, advantages of these multi-sectored transurethral applicators include a flexible delivery catheter and that mechanical manipulation of the device using rotational motors is not required during therapy. This multi-sectored tubular array transurethral ultrasound technology has demonstrated potential for relatively fast and reasonably conformal targeting of prostate volumes suitable for the minimally invasive treatment of BPH and cancer under MR guidance, with further development warranted.
Figures





Similar articles
-
Catheter-based ultrasound devices and MR thermal monitoring for conformal prostate thermal therapy.Annu Int Conf IEEE Eng Med Biol Soc. 2008;2008:3664-8. doi: 10.1109/IEMBS.2008.4650002. Annu Int Conf IEEE Eng Med Biol Soc. 2008. PMID: 19163505
-
Curvilinear transurethral ultrasound applicator for selective prostate thermal therapy.Med Phys. 2005 Jun;32(6):1555-65. doi: 10.1118/1.1924314. Med Phys. 2005. PMID: 16013714
-
Catheter-based ultrasound applicators for selective thermal ablation: progress towards MRI-guided applications in prostate.Int J Hyperthermia. 2004 Nov;20(7):739-56. doi: 10.1080/02656730410001721816. Int J Hyperthermia. 2004. PMID: 15675669 Review.
-
Transurethral ultrasound applicators with directional heating patterns for prostate thermal therapy: in vivo evaluation using magnetic resonance thermometry.Med Phys. 2004 Feb;31(2):405-13. doi: 10.1118/1.1639959. Med Phys. 2004. PMID: 15000627
-
Magnetic resonance-guided high-intensity ultrasound ablation of the prostate.Top Magn Reson Imaging. 2006 Jun;17(3):195-207. doi: 10.1097/RMR.0b013e31803774dd. Top Magn Reson Imaging. 2006. PMID: 17414077 Review.
Cited by
-
Development of robust/predictive control strategies for image-guided ablative treatments using a minimally invasive ultrasound applicator.Int J Hyperthermia. 2014 Nov;30(7):438-46. doi: 10.3109/02656736.2014.963702. Epub 2014 Oct 14. Int J Hyperthermia. 2014. PMID: 25314227 Free PMC article.
-
Considerations for theoretical modelling of thermal ablation with catheter-based ultrasonic sources: implications for treatment planning, monitoring and control.Int J Hyperthermia. 2012;28(1):69-86. doi: 10.3109/02656736.2011.630337. Int J Hyperthermia. 2012. PMID: 22235787 Free PMC article.
-
Focal ablation of prostate cancer: four roles for magnetic resonance imaging guidance.Can J Urol. 2013 Apr;20(2):6672-81. Can J Urol. 2013. PMID: 23587506 Free PMC article. Review.
-
Modelling of endoluminal and interstitial ultrasound hyperthermia and thermal ablation: applications for device design, feedback control and treatment planning.Int J Hyperthermia. 2013 Jun;29(4):296-307. doi: 10.3109/02656736.2013.800998. Int J Hyperthermia. 2013. PMID: 23738697 Free PMC article. Review.
-
Theoretical investigation of transgastric and intraductal approaches for ultrasound-based thermal therapy of the pancreas.J Ther Ultrasound. 2017 May 3;5:10. doi: 10.1186/s40349-017-0090-2. eCollection 2017. J Ther Ultrasound. 2017. PMID: 28469915 Free PMC article.
References
-
- Bruskewitz R., Issa M. M., Roehrborn C. G., Naslund M. J., Perez-Marrero R., Shumaker B. P., and Oesterling J. E., “A prospective, randomized 1-year clinical trial comparing transurethral needle ablation to transurethral resection of the prostate for the treatment of symptomatic benign prostatic hyperplasia,” J. Urol. 159, 1588–1593 (1998). - PubMed
-
- Alivizatos G., Ferakis N., Mitropoulos D., Skolarikos A., Livadas K., and Kastriotis I., “Feedback microwave thermotherapy with the ProstaLund Compact Device for obstructive benign prostatic hyperplasia: 12-month response rates and complications,” J. Endourol. 19, 72–78 (2005). - PubMed
-
- Larson B., Huidobro C., Acevedo C., Busel D., Mynderses L., Collins J., and Larson T., “In vivo temperature mapping of prostate during treatment with TherMatrx TMx-2000 device: Heat field and MRI determinations of necrotic lesions,” J. Endourol. 19, 1021–1025 (2005). - PubMed
-
- Sherar M. D., Trachtenberg J., Davidson S. R., McCann C., Yue C. K., Haider M. A., and Gertner M. R., “Interstitial microwave thermal therapy for prostate cancer,” J. Endourol. 17, 617–625 (2003). - PubMed
-
- Uchida T. et al. , “Transrectal high-intensity focused ultrasound in the treatment of localized prostate cancer: A multicenter study,” Hinyokika Kiyo 51, 651–658 (2005). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

