Retinoic acid regulates murine enteric nervous system precursor proliferation, enhances neuronal precursor differentiation, and reduces neurite growth in vitro
- PMID: 18561907
- PMCID: PMC2586054
- DOI: 10.1016/j.ydbio.2008.05.524
Retinoic acid regulates murine enteric nervous system precursor proliferation, enhances neuronal precursor differentiation, and reduces neurite growth in vitro
Abstract
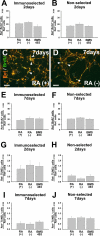
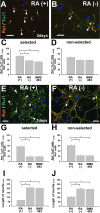
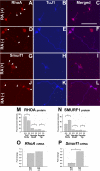
Enteric nervous system (ENS) precursors undergo a complex process of cell migration, proliferation, and differentiation to form an integrated network of neurons and glia within the bowel wall. Although retinoids regulate ENS development, molecular and cellular mechanisms of retinoid effects on the ENS are not well understood. We hypothesized that retinoids might directly affect ENS precursor differentiation and proliferation, and tested that hypothesis using immunoselected fetal ENS precursors in primary culture. We now demonstrate that all retinoid receptors and many retinoid biosynthetic enzymes are present in the fetal bowel at about the time that migrating ENS precursors reach the distal bowel. We further demonstrate that retinoic acid (RA) enhances proliferation of subsets of ENS precursors in a time-dependent fashion and increases neuronal differentiation. Surprisingly, however, enteric neurons that develop in retinoid deficient media have dramatically longer neurites than those exposed to RA. This difference in neurite growth correlates with increased RhoA protein at the neurite tip, decreased Smurf1 (a protein that targets RhoA for degradation), and dramatically decreased Smurf1 mRNA in response to RA. Collectively these data demonstrate diverse effects of RA on ENS precursor development and suggest that altered fetal retinoid availability or metabolism could contribute to intestinal motility disorders.
Figures





Similar articles
-
BMP signaling regulates murine enteric nervous system precursor migration, neurite fasciculation, and patterning via altered Ncam1 polysialic acid addition.Dev Biol. 2006 Nov 1;299(1):137-50. doi: 10.1016/j.ydbio.2006.07.016. Epub 2006 Jul 21. Dev Biol. 2006. PMID: 16952347 Free PMC article.
-
A novel bidirectional interaction between endothelin-3 and retinoic acid in rat enteric nervous system precursors.PLoS One. 2013 Sep 9;8(9):e74311. doi: 10.1371/journal.pone.0074311. eCollection 2013. PLoS One. 2013. PMID: 24040226 Free PMC article.
-
The timing and location of glial cell line-derived neurotrophic factor expression determine enteric nervous system structure and function.J Neurosci. 2010 Jan 27;30(4):1523-38. doi: 10.1523/JNEUROSCI.3861-09.2010. J Neurosci. 2010. PMID: 20107080 Free PMC article.
-
Pleiotropic effects of the bone morphogenetic proteins on development of the enteric nervous system.Dev Neurobiol. 2012 Jun;72(6):843-56. doi: 10.1002/dneu.22002. Dev Neurobiol. 2012. PMID: 22213745 Free PMC article. Review.
-
Death comes early: apoptosis observed in ENS precursors.Neurogastroenterol Motil. 2009 Jul;21(7):684-7. doi: 10.1111/j.1365-2982.2009.01325.x. Neurogastroenterol Motil. 2009. PMID: 19563403 Review.
Cited by
-
Cell-autonomous retinoic acid receptor signaling has stage-specific effects on mouse enteric nervous system.JCI Insight. 2021 May 24;6(10):e145854. doi: 10.1172/jci.insight.145854. JCI Insight. 2021. PMID: 33848271 Free PMC article.
-
Building a second brain in the bowel.J Clin Invest. 2015 Mar 2;125(3):899-907. doi: 10.1172/JCI76307. Epub 2015 Feb 9. J Clin Invest. 2015. PMID: 25664848 Free PMC article. Review.
-
Hirschsprung-like disease is exacerbated by reduced de novo GMP synthesis.J Clin Invest. 2013 Nov;123(11):4875-87. doi: 10.1172/JCI69781. J Clin Invest. 2013. PMID: 24216510 Free PMC article.
-
Neuroimmune regulation during intestinal development and homeostasis.Nat Immunol. 2017 Feb;18(2):116-122. doi: 10.1038/ni.3634. Epub 2017 Jan 16. Nat Immunol. 2017. PMID: 28092371 Review.
-
Enteric nervous system development: migration, differentiation, and disease.Am J Physiol Gastrointest Liver Physiol. 2013 Jul 1;305(1):G1-24. doi: 10.1152/ajpgi.00452.2012. Epub 2013 May 2. Am J Physiol Gastrointest Liver Physiol. 2013. PMID: 23639815 Free PMC article. Review.
References
-
- Baldassarre G, Boccia A, Bruni P, Sandomenico C, Barone MV, Pepe S, Angrisano T, Belletti B, Motti ML, Fusco A, Voglietto G. Retinoic acid induces neuronal differentiation of embryonal carcinoma cells by reducing proteasome-dependent proteolysis of the cyclin-dependent inhibitor p27. Cell Growth and Differentiation. 2000;11:517–526. - PubMed
-
- Barlow A, de Graaff E, Pachnis V. Enteric nervous system progenitors are coordinately controlled by the G protein-coupled receptor EDNRB and the receptor tyrosine kinase RET. Neuron. 2003;40:905–16. - PubMed
-
- Berggren K, McCaffery P, Drager U, Forehand CJ. Differential distribution of retinoic acid synthesis in the chicken embryo as determined by immunolocalization of the retinoic acid synthetic enzyme, RALDH-2. Dev Biol. 1999;210:288–304. - PubMed
-
- Bixby S, Kruger GM, Mosher JT, Joseph NM, Morrison SJ. Cell-intrinsic differences between stem cells from different regions of the peripheral nervous system regulate the generation of neural diversity. Neuron. 2002;35:643–56. - PubMed
-
- Blomhoff R, Blomhoff HK. Overview of retinoid metabolism and function. J Neurobiol. 2006;66:606–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

