Branch architecture of the fly larval abdominal serotonergic neurons
- PMID: 18561908
- PMCID: PMC2610461
- DOI: 10.1016/j.ydbio.2008.03.038
Branch architecture of the fly larval abdominal serotonergic neurons
Abstract
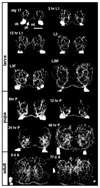
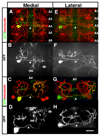
In the metazoan central nervous system (CNS), serotonergic neurons send projections throughout the synaptic neuropil. Little is known about the rules that govern these widespread neuromodulatory branching patterns. In this study, we utilize the Drosophila as a model to examine serotonergic branching. Using single cell GFP labeling we show that within each segment of the Drosophila ventral nerve cord (VNC), each of two serotonergic neurons tiles distinct innervation patterns in the contralateral neuropil. In addition, branches extend only a short distance from the target segment. Through ablation-mediated isolation of serotonergic cells, we demonstrate that the distinct areas of innervation are not maintained through competition between neighboring like-serotonergic neurites. Furthermore, the basic branching pattern of serotonergic neurons within the neuropil remains unchanged despite alterations of initial axonal trajectories.
Figures



References
-
- Bonkowsky JL, Yoshikawa S, O'Keefe DD, Scully AL, Thomas JB. Axon routing across the midline controlled by the Drosophila Derailed receptor. Nature. 1999;402:540–544. - PubMed
-
- Daubert EA, Condron BG. A Solid-Phase Immunostaining Protocol for High-Resolution Imaging of Delicate Structures in the Drosophila Larval Central Nervous System. CSH protocols. 2007 - PubMed
-
- Dittrich R, Bossing T, Gould AP, Technau GM, Urban J. The differentiation of the serotonergic neurons in the Drosophila ventral nerve cord depends on the combined function of the zinc finger proteins Eagle and Huckebein. Development. 1997;124:2515–2525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

