Diminished mitochondrial DNA integrity and repair capacity in OA chondrocytes
- PMID: 18562218
- PMCID: PMC3640312
- DOI: 10.1016/j.joca.2008.05.009
Diminished mitochondrial DNA integrity and repair capacity in OA chondrocytes
Abstract
Objectives: Osteoarthritis (OA) is characterized by the failure of chondrocytes to respond to injury and perform the cartilage remodeling process. Human articular chondrocytes actively produce reactive oxygen and nitrogen species (ROS and RNS) capable of causing cellular dysfunction and death. A growing body of evidence indicates that mitochondrial dysfunction and mitochondrial DNA (mtDNA) damage play a causal role in disorders linked to excessive generation of oxygen free radicals. The aim of this study was to determine whether mtDNA damage was present in OA chondrocytes, and whether mtDNA repair capacity is compromised in OA chondrocytes following oxidative stress, leading to chondrocyte death.
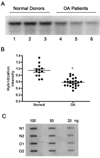
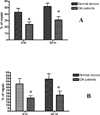
Methods: Human articular cartilage was isolated from knee joints of cadavers available through the Anatomical Gifts Program at the University of South Alabama (normal donors) or OA patients undergoing total knee replacement surgeries (OA patients). Total DNA was isolated from either chondrocytes released following collagenase digestion, or from first passage chondrocytes grown in culture and exposed to ROS or RNS. mtDNA integrity and repair capacity were analyzed by quantitative Southern blot analysis, using a mtDNA-specific radioactive probe. Cell viability was determined by the trypan blue exclusion method.
Results: mtDNA damage was found in chondrocytes from OA patients compared to normal donors. It was accompanied with reduced mtDNA repair capacity, cell viability, and increased apoptosis in OA chondrocytes following exposure to ROS and RNS.
Conclusions: These results indicate that mtDNA damage and poor mtDNA repair capacity for removing damage caused by oxidative stress may contribute to the pathogenesis of OA.
Figures



References
-
- Henrotin YE, Bruckner P, Pujol JP. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthritis Cartilage. 2003;11:747–755. - PubMed
-
- Mazzetti I, Grigolo B, Pulsatelli L, Dolzani P, Silvestri T, Roseti L, et al. Differential roles of nitric oxide and oxygen radicals in chondrocytes affected by osteoarthritis and rheumatoid arthritis. Clin Sci (Lond) 2001;101:593–599. - PubMed
-
- Fermor B, Weinberg JB, Pisetsky DS, Misukonis MA, Banes AJ, Guilak F. The effects of static and intermittent compression on nitric oxide production in articular cartilage explants. J Orthop Res. 2001;19:729–737. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

