Ablation of thymic export causes accelerated decay of naive CD4 T cells in the periphery because of activation by environmental antigen
- PMID: 18562288
- PMCID: PMC2438401
- DOI: 10.1073/pnas.0803732105
Ablation of thymic export causes accelerated decay of naive CD4 T cells in the periphery because of activation by environmental antigen
Abstract
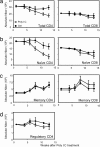
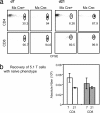
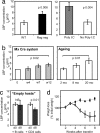
A model of chemical thymectomy by inducible Rag ablation was used to study peripheral T cell homeostasis. Induction of Rag ablation was efficient and complete, leading to cessation of thymic T cell production within 3-4 weeks. The decay of peripheral T cells became apparent with a delay of an additional 2-3 weeks and was entirely accounted for by loss of naïve T cells, whereas numbers of memory phenotype and regulatory T cells were not decreased. Naïve CD4 T cells decayed with an average half-life of 50 days, whereas naïve CD8 T cells exhibited a considerably longer half-life. The rapid decay of naïve CD4 T cells was not caused by intrinsic survival differences compared with naïve CD8 T cells, but was caused by changes in the lymphopenic environment resulting in higher microbial load and consequential activation. This finding suggests that in lymphopenic conditions involving compromised thymic function replenishment and survival of a naïve CD4 T cell repertoire may be severely curtailed because of chronic activation. Such a scenario might play a role in the aging immune system and chronic viral infection, such as HIV infection, and contribute to loss of CD4 T cells and impaired immune function. As our data show, continued replenishment with cells from the thymus seems to be required to maintain efficient gut mucosal defense.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Tanchot C, Rocha B. The peripheral T cell repertoire: Independent homeostatic regulation of virgin and activated CD8+ T cell pools. Eur J Immunol. 1995;25:2127–2136. - PubMed
-
- Jameson SC. Maintaining the norm: T cell homeostasis. Nat Rev Immunol. 2002;2:547–556. - PubMed
-
- Mackall CL, Gress RE. Thymic aging and T cell regeneration. Immunol Rev. 1997;160:91–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

