Apolipoprotein (apo) E4 enhances HIV-1 cell entry in vitro, and the APOE epsilon4/epsilon4 genotype accelerates HIV disease progression
- PMID: 18562290
- PMCID: PMC2438419
- DOI: 10.1073/pnas.0803526105
Apolipoprotein (apo) E4 enhances HIV-1 cell entry in vitro, and the APOE epsilon4/epsilon4 genotype accelerates HIV disease progression
Abstract
Originally recognized for their role in lipoprotein metabolism and cardiovascular disease, apolipoprotein (apo) E isoforms (apoE2, apoE3, and apoE4) have also been implicated to play a key role in several biological processes not directly related to their lipid transport function. For example, apoE4 contributes significantly to neurodegeneration in Alzheimer's disease. However, the role of apoE in infectious diseases is less well defined. Here, by examining a large cohort of HIV(+) European and African American subjects, we found that the APOE epsilon4/epsilon4 genotype is associated with an accelerated disease course and especially progression to death compared with the APOE epsilon3/epsilon3 genotype. However, an association between the epsilon4/epsilon4 genotype and HIV-associated dementia (HAD), a neurological condition with clinicopathological features similar to Alzheimer's disease, was not detected. Consistent with the genotype-phenotype relationships observed, compared with recombinant apoE3, apoE4 enhanced HIV fusion/cell entry of both R5 and X4 HIV strains in vitro. These findings establish apoE as a determinant of HIV-AIDS pathogenesis and raise the possibility that current efforts to convert apoE4 to an "apoE3-like" molecule to treat Alzheimer's disease might also have clinical applicability in HIV disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
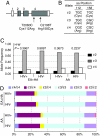
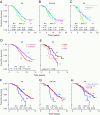
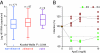
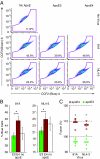
Comment in
-
Deleterious CNS effects of the APOE epsilon4 allele in individuals with HIV-1 infection may be age-dependent.Proc Natl Acad Sci U S A. 2008 Oct 14;105(41):E65; author reply E67-8. doi: 10.1073/pnas.0806356105. Epub 2008 Oct 8. Proc Natl Acad Sci U S A. 2008. PMID: 18843100 Free PMC article. No abstract available.
-
Frequency of apolipoprotein E4 among older compared with younger HIV patients: support for detrimental effect of E4 on survival.Proc Natl Acad Sci U S A. 2008 Oct 14;105(41):E66; author reply E67-8. doi: 10.1073/pnas.0806919105. Epub 2008 Oct 8. Proc Natl Acad Sci U S A. 2008. PMID: 18843101 Free PMC article. No abstract available.
-
Differential course of HIV-1 infection and APOE polymorphism.Proc Natl Acad Sci U S A. 2008 Nov 18;105(46):E87. doi: 10.1073/pnas.0808164105. Epub 2008 Nov 11. Proc Natl Acad Sci U S A. 2008. PMID: 19004794 Free PMC article. No abstract available.
References
-
- Kaslow RA, Dorak T, Tang JJ. Influence of host genetic variation on susceptibility to HIV type 1 infection. J Infect Dis. 2005;191:S68–S77. - PubMed
-
- Fatkenheuer G, et al. Efficacy of short-term monotherapy with maraviroc, a new CCR5 antagonist, in patients infected with HIV-1. Nat Med. 2005;11:1170–1172. - PubMed
-
- Mahley RW, Rall SC., Jr Apolipoprotein E: Far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–537. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

