Resveratrol inhibits cardiac hypertrophy via AMP-activated protein kinase and Akt
- PMID: 18562309
- PMCID: PMC3259789
- DOI: 10.1074/jbc.M802869200
Resveratrol inhibits cardiac hypertrophy via AMP-activated protein kinase and Akt
Abstract
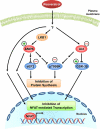
Whereas studies involving animal models of cardiovascular disease demonstrated that resveratrol is able to inhibit hypertrophic growth, the mechanisms involved have not been elucidated. Because studies in cells other than cardiomyocytes revealed that AMP-activated protein kinase (AMPK) and Akt are affected by resveratrol, we hypothesized that resveratrol prevents cardiac myocyte hypertrophy via these two kinase systems. Herein, we demonstrate that resveratrol reduces phenylephrine-induced protein synthesis and cell growth in rat cardiac myocytes via alterations of intracellular pathways involved in controlling protein synthesis (p70S6 kinase and eukaryotic elongation factor-2). Additionally, we demonstrate that resveratrol negatively regulates the calcineurin-nuclear factor of activated T cells pathway thus modifying a critical component of the transcriptional mechanism involved in pathological cardiac hypertrophy. Our data also indicate that these effects of resveratrol are mediated via AMPK activation and Akt inhibition, and in the case of AMPK, is dependent on the presence of the AMPK kinase, LKB1. Taken together, our data suggest that resveratrol exerts anti-hypertrophic effects by activating AMPK via LKB1 and inhibiting Akt, thus suppressing protein synthesis and gene transcription.
Figures
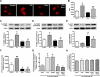
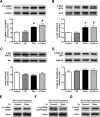
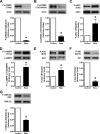
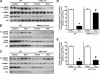
Similar articles
-
Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte.J Biol Chem. 2004 Jul 30;279(31):32771-9. doi: 10.1074/jbc.M403528200. Epub 2004 May 24. J Biol Chem. 2004. PMID: 15159410
-
Resveratrol prevents the prohypertrophic effects of oxidative stress on LKB1.Circulation. 2009 Mar 31;119(12):1643-52. doi: 10.1161/CIRCULATIONAHA.108.787440. Epub 2009 Mar 16. Circulation. 2009. PMID: 19289642
-
Long-term activation of adenosine monophosphate-activated protein kinase attenuates pressure-overload-induced cardiac hypertrophy.J Cell Biochem. 2007 Apr 1;100(5):1086-99. doi: 10.1002/jcb.21197. J Cell Biochem. 2007. PMID: 17266062
-
Activation of AMP-activated protein kinase (AMPK) inhibits protein synthesis: a potential strategy to prevent the development of cardiac hypertrophy.Can J Physiol Pharmacol. 2005 Jan;83(1):24-8. doi: 10.1139/y04-107. Can J Physiol Pharmacol. 2005. PMID: 15759047 Review.
-
AMPK alterations in cardiac physiology and pathology: enemy or ally?J Physiol. 2006 Jul 1;574(Pt 1):95-112. doi: 10.1113/jphysiol.2006.109389. Epub 2006 May 11. J Physiol. 2006. PMID: 16690706 Free PMC article. Review.
Cited by
-
Mitochondrial quality control in health and cardiovascular diseases.Front Cell Dev Biol. 2023 Nov 6;11:1290046. doi: 10.3389/fcell.2023.1290046. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38020895 Free PMC article. Review.
-
Resveratrol Ameliorates Cardiac Hypertrophy by Down-regulation of miR-155 Through Activation of Breast Cancer Type 1 Susceptibility Protein.J Am Heart Assoc. 2016 Apr 22;5(4):e002648. doi: 10.1161/JAHA.115.002648. J Am Heart Assoc. 2016. PMID: 27107135 Free PMC article.
-
Targeting mitochondria for cardiovascular disorders: therapeutic potential and obstacles.Nat Rev Cardiol. 2019 Jan;16(1):33-55. doi: 10.1038/s41569-018-0074-0. Nat Rev Cardiol. 2019. PMID: 30177752 Free PMC article. Review.
-
Exogenous NAD blocks cardiac hypertrophic response via activation of the SIRT3-LKB1-AMP-activated kinase pathway.J Biol Chem. 2010 Jan 29;285(5):3133-44. doi: 10.1074/jbc.M109.077271. Epub 2009 Nov 24. J Biol Chem. 2010. PMID: 19940131 Free PMC article.
-
Natural AMPK Activators in Cardiovascular Disease Prevention.Front Pharmacol. 2022 Jan 3;12:738420. doi: 10.3389/fphar.2021.738420. eCollection 2021. Front Pharmacol. 2022. PMID: 35046800 Free PMC article. Review.
References
-
- Benjamin, E. J., and Levy, D. (1999) Am. J. Med. Sci. 317 168-175 - PubMed
-
- Wilkins, B. J., Dai, Y. S., Bueno, O. F., Parsons, S. A., Xu, J., Plank, D. M., Jones, F., Kimball, T. R., and Molkentin, J. D. (2004) Circ. Res. 94 110-118 - PubMed
-
- Wilkins, B. J., and Molkentin, J. D. (2004) Biochem. Biophys. Res. Commun. 322 1178-1191 - PubMed
-
- Horman, S., Beauloye, C., Vertommen, D., Vanoverschelde, J. L., Hue, L., and Rider, M. H. (2003) J. Biol. Chem. 278 41970-41976 - PubMed
-
- Chan, A. Y., Soltys, C. L., Young, M. E., Proud, C. G., and Dyck, J. R. (2004) J. Biol. Chem. 279 32771-32779 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

