Intermediate phosphorylation reactions in the mechanism of ATP utilization by the copper ATPase (CopA) of Thermotoga maritima
- PMID: 18562314
- PMCID: PMC2504886
- DOI: 10.1074/jbc.M802735200
Intermediate phosphorylation reactions in the mechanism of ATP utilization by the copper ATPase (CopA) of Thermotoga maritima
Abstract
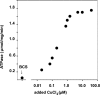
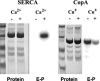
Recombinant and purified Thermotoga maritima CopA sustains ATPase velocity of 1.78-2.73 micromol/mg/min in the presence of Cu+ (pH 6, 60 degrees C) and 0.03-0.08 micromol/mg/min in the absence of Cu+. High levels of enzyme phosphorylation are obtained by utilization of [gamma-32P]ATP in the absence of Cu+. This phosphoenzyme decays at a much slower rate than observed with Cu.E1 approximately P. In fact, the phosphoenzyme is reduced to much lower steady state levels upon addition of Cu+, due to rapid hydrolytic cleavage. Negligible ATPase turnover is sustained by CopA following deletion of its N-metal binding domain (DeltaNMBD) or mutation of NMBD cysteines (CXXC). Nevertheless, high levels of phosphoenzyme are obtained by utilization of [gamma-3)P]ATP by the DeltaNMBD and CXXC mutants, with no effect of Cu+ either on its formation or hydrolytic cleavage. Phosphoenzyme formation (E2P) can also be obtained by utilization of Pi, and this reaction is inhibited by Cu+ (E2 to E1 transition) even in the DeltaNMBD mutant, evidently due to Cu+ binding at a (transport) site other than the NMBD. E2P undergoes hydrolytic cleavage faster in DeltaNMBD and slower in CXXC mutant. We propose that Cu+ binding to the NMBD is required to produce an "active" conformation of CopA, whereby additional Cu+ bound to an alternate (transmembrane transport) site initiates faster cycles including formation of Cu.E1 approximately P, followed by the E1 approximately P to E2-P conformational transition and hydrolytic cleavage of phosphate. An H479Q mutation (analogous to one found in Wilson disease) renders CopA unable to utilize ATP, whereas phosphorylation by Pi is retained.
Figures











Similar articles
-
Reaction cycle of Thermotoga maritima copper ATPase and conformational characterization of catalytically deficient mutants.Biochemistry. 2009 Jun 9;48(22):4871-80. doi: 10.1021/bi900338n. Biochemistry. 2009. PMID: 19364131 Free PMC article.
-
Domain organization and movements in heavy metal ion pumps: papain digestion of CopA, a Cu+-transporting ATPase.J Biol Chem. 2007 Aug 31;282(35):25213-21. doi: 10.1074/jbc.M703520200. Epub 2007 Jul 6. J Biol Chem. 2007. PMID: 17616523
-
Biochemical characterization of CopA, the Escherichia coli Cu(I)-translocating P-type ATPase.J Biol Chem. 2002 Dec 6;277(49):46987-92. doi: 10.1074/jbc.M208490200. Epub 2002 Sep 25. J Biol Chem. 2002. PMID: 12351646
-
Biochemical characterization of P-type copper ATPases.Biochem J. 2014 Oct 15;463(2):167-76. doi: 10.1042/BJ20140741. Biochem J. 2014. PMID: 25242165 Free PMC article. Review.
-
Mechanisms of charge transfer in human copper ATPases ATP7A and ATP7B.IUBMB Life. 2017 Apr;69(4):218-225. doi: 10.1002/iub.1603. Epub 2017 Feb 5. IUBMB Life. 2017. PMID: 28164426 Review.
Cited by
-
Mycobacterial resistance to zinc poisoning requires assembly of P-ATPase-containing membrane metal efflux platforms.Nat Commun. 2022 Aug 12;13(1):4731. doi: 10.1038/s41467-022-32085-7. Nat Commun. 2022. PMID: 35961955 Free PMC article.
-
The architecture of CopA from Archeaoglobus fulgidus studied by cryo-electron microscopy and computational docking.Structure. 2011 Sep 7;19(9):1219-32. doi: 10.1016/j.str.2011.05.014. Epub 2011 Aug 4. Structure. 2011. PMID: 21820315 Free PMC article.
-
Copper-transporting P-type ATPases use a unique ion-release pathway.Nat Struct Mol Biol. 2014 Jan;21(1):43-8. doi: 10.1038/nsmb.2721. Epub 2013 Dec 8. Nat Struct Mol Biol. 2014. PMID: 24317491 Free PMC article.
-
The transport mechanism of bacterial Cu+-ATPases: distinct efflux rates adapted to different function.Biometals. 2011 Jun;24(3):467-75. doi: 10.1007/s10534-010-9404-3. Epub 2011 Jan 6. Biometals. 2011. PMID: 21210186 Free PMC article.
-
An NMR study of the interaction of the N-terminal cytoplasmic tail of the Wilson disease protein with copper(I)-HAH1.J Biol Chem. 2009 Apr 3;284(14):9354-60. doi: 10.1074/jbc.M805981200. Epub 2009 Jan 30. J Biol Chem. 2009. PMID: 19181666 Free PMC article.
References
-
- Pedersen, P. L., and Carafoli, E. (1987) Trends Biochem. Sci. 12 146-150
-
- Lutsenko, S., and Kaplan, J. H. (1995) Biochemistry 34 15607-15613 - PubMed
-
- Moller, J. V., Juul, B., and Le Maire, M. (1996) Biochim. Biophys. Acta 1286 1-51 - PubMed
-
- Axelsen, K. B., and Palmgern, M. G. (1998) J. Mol. Evol. 46 84-101 - PubMed
-
- Arguello, J. M. (2003) J. Membr. Biol. 195 93-1008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

