Pregnancy-upregulated nonubiquitous calmodulin kinase induces ligand-independent EGFR degradation
- PMID: 18562482
- PMCID: PMC2518423
- DOI: 10.1152/ajpcell.00449.2007
Pregnancy-upregulated nonubiquitous calmodulin kinase induces ligand-independent EGFR degradation
Abstract
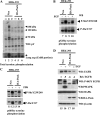
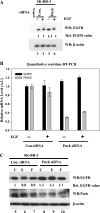
We describe here an important function of the novel calmodulin kinase I isoform, pregnancy-upregulated nonubiquitous calmodulin kinase (Pnck). Pnck (also known as CaM kinase Ibeta(2)) was previously shown to be differentially overexpressed in a subset of human primary breast cancers, compared with benign mammary epithelial tissue. In addition, during late pregnancy, Pnck mRNA was shown to be strongly upregulated in epithelial cells of the mouse mammary gland exhibiting decreased proliferation and terminal differentiation. Pnck mRNA is also significantly upregulated in confluent and serum-starved cells, compared with actively growing proliferating cells (Gardner HP, Seung HI, Reynolds C, Chodosh LA. Cancer Res 60: 5571-5577, 2000). Despite these suggestive data, the true physiological role(s) of, or the signaling mechanism(s) regulated by Pnck, remain unknown. We now report that epidermal growth factor receptor (EGFR) levels are significantly downregulated in a ligand-independent manner in human embryonic kidney-293 (HEK-293) cells overexpressing Pnck. MAP kinase activation was strongly inhibited by EGFR downregulation in the Pnck-overexpressing cells. The EGFR downregulation was not the result of reduced transcription of the EGFR gene but from protea-lysosomal degradation of EGFR protein. Knockdown of endogenous Pnck mRNA levels by small interfering RNA transfection in human breast cancer cells resulted in upregulation of unliganded EGFR, consistent with the effects observed in the overexpression model of Pnck-mediated ligand-independent EGFR downregulation. Pnck thus emerges as a new component of the poorly understood mechanism of ligand-independent EGFR degradation, and it may represent an attractive therapeutic target in EGFR-regulated oncogenesis.
Figures




Similar articles
-
Pnck induces ligand-independent EGFR degradation by probable perturbation of the Hsp90 chaperone complex.Am J Physiol Cell Physiol. 2011 May;300(5):C1139-54. doi: 10.1152/ajpcell.00167.2010. Epub 2011 Feb 16. Am J Physiol Cell Physiol. 2011. PMID: 21325639 Free PMC article.
-
PTEN-mediated ERK1/2 inhibition and paradoxical cellular proliferation following Pnck overexpression.Cell Cycle. 2014;13(6):961-73. doi: 10.4161/cc.27837. Epub 2014 Jan 20. Cell Cycle. 2014. PMID: 24552815 Free PMC article.
-
Pnck overexpression in HER-2 gene-amplified breast cancer causes Trastuzumab resistance through a paradoxical PTEN-mediated process.Breast Cancer Res Treat. 2015 Apr;150(2):347-61. doi: 10.1007/s10549-015-3337-z. Epub 2015 Mar 15. Breast Cancer Res Treat. 2015. PMID: 25773930
-
The caM kinase, Pnck, is spatially and temporally regulated during murine mammary gland development and may identify an epithelial cell subtype involved in breast cancer.Cancer Res. 2000 Oct 1;60(19):5571-7. Cancer Res. 2000. PMID: 11034105
-
Ligand regulates epidermal growth factor receptor kinase specificity: activation increases preference for GAB1 and SHC versus autophosphorylation sites.J Biol Chem. 2004 Sep 10;279(37):38143-50. doi: 10.1074/jbc.M405760200. Epub 2004 Jul 1. J Biol Chem. 2004. PMID: 15231819
Cited by
-
PNCK depletion inhibits proliferation and induces apoptosis of human nasopharyngeal carcinoma cells in vitro and in vivo.J Cancer. 2019 Dec 3;10(27):6925-6932. doi: 10.7150/jca.33698. eCollection 2019. J Cancer. 2019. PMID: 31839828 Free PMC article.
-
Increased expression of pregnancy up-regulated non-ubiquitous calmodulin kinase is associated with poor prognosis in clear cell renal cell carcinoma.PLoS One. 2013 Apr 25;8(4):e59936. doi: 10.1371/journal.pone.0059936. Print 2013. PLoS One. 2013. PMID: 23634203 Free PMC article.
-
A novel intergenic ETnII-β insertion mutation causes multiple malformations in polypodia mice.PLoS Genet. 2013;9(12):e1003967. doi: 10.1371/journal.pgen.1003967. Epub 2013 Dec 5. PLoS Genet. 2013. PMID: 24339789 Free PMC article.
-
Pnck induces ligand-independent EGFR degradation by probable perturbation of the Hsp90 chaperone complex.Am J Physiol Cell Physiol. 2011 May;300(5):C1139-54. doi: 10.1152/ajpcell.00167.2010. Epub 2011 Feb 16. Am J Physiol Cell Physiol. 2011. PMID: 21325639 Free PMC article.
-
Integrated profiling of microRNAs and mRNAs: microRNAs located on Xq27.3 associate with clear cell renal cell carcinoma.PLoS One. 2010 Dec 30;5(12):e15224. doi: 10.1371/journal.pone.0015224. PLoS One. 2010. PMID: 21253009 Free PMC article.
References
-
- Alwan HA, van Zoelen EJ, van Leeuwen JE. Ligand-induced lysosomal epidermal growth factor receptor (EGFR) degradation is preceded by proteasome-dependent EGFR de-ubiquitination. J Biol Chem 278: 35781–35790, 2003. - PubMed
-
- Baselga J, Mendelsohn J. The epidermal growth factor receptor as a target for therapy in breast carcinoma. Breast Cancer Res Treat 29: 127–138, 1994. - PubMed
-
- Bowtell DD, Langdon WY. The protein product of the c-cbl oncogene rapidly complexes with the EGF receptor and is tyrosine phosphorylated following EGF stimulation. Oncogene 11: 1561–1567, 1995. - PubMed
-
- Callus BA, Mathey-Prevot B. SOCS36E, a novel Drosophila SOCS protein, suppresses JAK/STAT and EGF-R signalling in the imaginal wing disc. Oncogene 21: 4812–4821, 2002. - PubMed
-
- Carlin CR, Tollefson AE, Brady HA, Hoffman BL, Wold WS. Epidermal growth factor receptor is down-regulated by a 10,400 MW protein encoded by the E3 region of adenovirus. Cell 57: 135–144, 1989. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

