Direct regulation of BK channels by phosphatidylinositol 4,5-bisphosphate as a novel signaling pathway
- PMID: 18562499
- PMCID: PMC2442183
- DOI: 10.1085/jgp.200709913
Direct regulation of BK channels by phosphatidylinositol 4,5-bisphosphate as a novel signaling pathway
Abstract
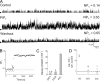
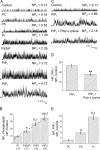
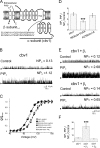
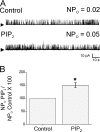
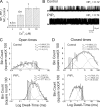
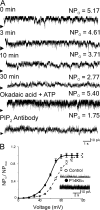
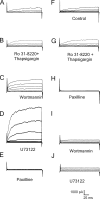
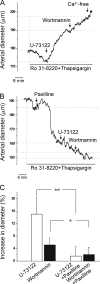
Large conductance, calcium- and voltage-gated potassium (BK) channels are ubiquitous and critical for neuronal function, immunity, and smooth muscle contractility. BK channels are thought to be regulated by phosphatidylinositol 4,5-bisphosphate (PIP(2)) only through phospholipase C (PLC)-generated PIP(2) metabolites that target Ca(2+) stores and protein kinase C and, eventually, the BK channel. Here, we report that PIP(2) activates BK channels independently of PIP(2) metabolites. PIP(2) enhances Ca(2+)-driven gating and alters both open and closed channel distributions without affecting voltage gating and unitary conductance. Recovery from activation was strongly dependent on PIP(2) acyl chain length, with channels exposed to water-soluble diC4 and diC8 showing much faster recovery than those exposed to PIP(2) (diC16). The PIP(2)-channel interaction requires negative charge and the inositol moiety in the phospholipid headgroup, and the sequence RKK in the S6-S7 cytosolic linker of the BK channel-forming (cbv1) subunit. PIP(2)-induced activation is drastically potentiated by accessory beta(1) (but not beta(4)) channel subunits. Moreover, PIP(2) robustly activates BK channels in vascular myocytes, where beta(1) subunits are abundantly expressed, but not in skeletal myocytes, where these subunits are barely detectable. These data demonstrate that the final PIP(2) effect is determined by channel accessory subunits, and such mechanism is subunit specific. In HEK293 cells, cotransfection of cbv1+beta(1) and PI4-kinaseIIalpha robustly activates BK channels, suggesting a role for endogenous PIP(2) in modulating channel activity. Indeed, in membrane patches excised from vascular myocytes, BK channel activity runs down and Mg-ATP recovers it, this recovery being abolished by PIP(2) antibodies applied to the cytosolic membrane surface. Moreover, in intact arterial myocytes under physiological conditions, PLC inhibition on top of blockade of downstream signaling leads to drastic BK channel activation. Finally, pharmacological treatment that raises PIP(2) levels and activates BK channels dilates de-endothelized arteries that regulate cerebral blood flow. These data indicate that endogenous PIP(2) directly activates vascular myocyte BK channels to control vascular tone.
Figures

Comment in
-
PIP2 PIP2 hooray for maxi K+.J Gen Physiol. 2008 Jul;132(1):5-8. doi: 10.1085/jgp.200810053. Epub 2008 Jun 18. J Gen Physiol. 2008. PMID: 18562503 Free PMC article. No abstract available.
References
-
- Barman, S., S. Zhu, and R. White. 2004. PKC activates BKCa channels in rat pulmonary arterial smooth muscle via cGMP-dependent protein kinase. Am. J. Physiol. Lung Cell Mol. Physiol. 286:L149–L155. - PubMed
-
- Behrens, R., A. Nolting, F. Reimann, M. Schwarz, R. Waldschutz, and O. Pongs. 2000. hKCNMB3 and hKCNMB4, cloning and characterization of two members of the large-conductance calcium-activated potassium channel β subunit family. FEBS Lett. 474:99–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

