Nonstructural proteins 1 and 2 of respiratory syncytial virus suppress maturation of human dendritic cells
- PMID: 18562519
- PMCID: PMC2519638
- DOI: 10.1128/JVI.00630-08
Nonstructural proteins 1 and 2 of respiratory syncytial virus suppress maturation of human dendritic cells
Abstract
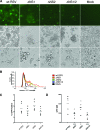
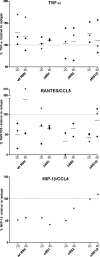
Human respiratory syncytial virus (RSV) is the most important agent of serious pediatric respiratory tract disease worldwide. One of the main characteristics of RSV is that it readily reinfects and causes disease throughout life without the need for significant antigenic change. The virus encodes nonstructural protein 1 (NS1) and NS2, which are known to suppress type I interferon (IFN) production and signaling. In the present study, we monitored the maturation of human monocyte-derived myeloid dendritic cells (DC) following inoculation with recombinant RSVs bearing deletions of the NS1 and/or NS2 proteins and expressing enhanced green fluorescent protein. Deletion of the NS1 protein resulted in increased expression of cell surface markers of DC maturation and an increase in the expression of multiple cytokines and chemokines. This effect was enhanced somewhat by further deletion of the NS2 protein, although deletion of NS2 alone did not have a significant effect. The upregulation was largely inhibited by pretreatment with a blocking antibody against the type I IFN receptor, suggesting that suppression of DC maturation by NS1/2 is, at least in part, a result of IFN antagonism mediated by these proteins. Therefore, this study identified another effect of the NS1 and NS2 proteins. The observed suppression of DC maturation may result in decreased antigen presentation and T-lymphocyte activation, leading to incomplete and/or weak immune responses that might contribute to RSV reinfection.
Figures





Similar articles
-
Effects of nonstructural proteins NS1 and NS2 of human respiratory syncytial virus on interferon regulatory factor 3, NF-kappaB, and proinflammatory cytokines.J Virol. 2005 May;79(9):5353-62. doi: 10.1128/JVI.79.9.5353-5362.2005. J Virol. 2005. PMID: 15827150 Free PMC article.
-
Replacement of the respiratory syncytial virus nonstructural proteins NS1 and NS2 by the V protein of parainfluenza virus 5.Virology. 2007 Nov 10;368(1):73-82. doi: 10.1016/j.virol.2007.06.017. Epub 2007 Jul 16. Virology. 2007. PMID: 17632199 Free PMC article.
-
Lack of Activation Marker Induction and Chemokine Receptor Switch in Human Neonatal Myeloid Dendritic Cells in Response to Human Respiratory Syncytial Virus.J Virol. 2019 Oct 29;93(22):e01216-19. doi: 10.1128/JVI.01216-19. Print 2019 Nov 15. J Virol. 2019. PMID: 31484754 Free PMC article.
-
Respiratory syncytial virus nonstructural proteins 1 and 2: Exceptional disrupters of innate immune responses.PLoS Pathog. 2019 Oct 17;15(10):e1007984. doi: 10.1371/journal.ppat.1007984. eCollection 2019 Oct. PLoS Pathog. 2019. PMID: 31622448 Free PMC article. Review.
-
An Unexpected Encounter: Respiratory Syncytial Virus Nonstructural Protein 1 Interacts with Mediator Subunit MED25.J Virol. 2022 Oct 12;96(19):e0129722. doi: 10.1128/jvi.01297-22. Epub 2022 Sep 14. J Virol. 2022. PMID: 36102648 Free PMC article. Review.
Cited by
-
PA-X protein of H1N1 subtype influenza virus disables the nasal mucosal dendritic cells for strengthening virulence.Virulence. 2022 Dec;13(1):1928-1942. doi: 10.1080/21505594.2022.2139474. Virulence. 2022. PMID: 36271710 Free PMC article.
-
Human parainfluenza virus type 3 expressing the respiratory syncytial virus pre-fusion F protein modified for virion packaging yields protective intranasal vaccine candidates.PLoS One. 2020 Feb 11;15(2):e0228572. doi: 10.1371/journal.pone.0228572. eCollection 2020. PLoS One. 2020. PMID: 32045432 Free PMC article.
-
Neonatal Immunity, Respiratory Virus Infections, and the Development of Asthma.Front Immunol. 2018 Jun 4;9:1249. doi: 10.3389/fimmu.2018.01249. eCollection 2018. Front Immunol. 2018. PMID: 29915592 Free PMC article. Review.
-
Tick-borne flaviviruses antagonize both IRF-1 and type I IFN signaling to inhibit dendritic cell function.J Immunol. 2014 Mar 15;192(6):2744-55. doi: 10.4049/jimmunol.1302110. Epub 2014 Feb 14. J Immunol. 2014. PMID: 24532583 Free PMC article.
-
Immune-Modulation by the Human Respiratory Syncytial Virus: Focus on Dendritic Cells.Front Immunol. 2019 Apr 15;10:810. doi: 10.3389/fimmu.2019.00810. eCollection 2019. Front Immunol. 2019. PMID: 31057543 Free PMC article. Review.
References
-
- Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392245-252. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

