Cathepsin L functionally cleaves the severe acute respiratory syndrome coronavirus class I fusion protein upstream of rather than adjacent to the fusion peptide
- PMID: 18562523
- PMCID: PMC2519682
- DOI: 10.1128/JVI.00415-08
Cathepsin L functionally cleaves the severe acute respiratory syndrome coronavirus class I fusion protein upstream of rather than adjacent to the fusion peptide
Abstract
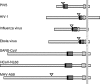
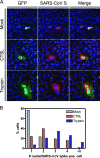
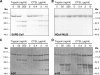
Unlike other class I viral fusion proteins, spike proteins on severe acute respiratory syndrome coronavirus virions are uncleaved. As we and others have demonstrated, infection by this virus depends on cathepsin proteases present in endosomal compartments of the target cell, suggesting that the spike protein acquires its fusion competence by cleavage during cell entry rather than during virion biogenesis. Here we demonstrate that cathepsin L indeed activates the membrane fusion function of the spike protein. Moreover, cleavage was mapped to the same region where, in coronaviruses carrying furin-activated spikes, the receptor binding subunit of the protein is separated from the membrane-anchored fusion subunit.
Figures

References
-
- Bosch, B. J., B. E. Martina, R. Van Der Zee, J. Lepault, B. J. Haijema, C. Versluis, A. J. Heck, R. De Groot, A. D. Osterhaus, and P. J. Rottier. 2004. Severe acute respiratory syndrome coronavirus (SARS-CoV) infection inhibition using spike protein heptad repeat-derived peptides. Proc. Natl. Acad. Sci. USA 1018455-8460. - PMC - PubMed
-
- Calder, L. J., L. Gonzalez-Reyes, B. Garcia-Barreno, S. A. Wharton, J. J. Skehel, D. C. Wiley, and J. A. Melero. 2000. Electron microscopy of the human respiratory syncytial virus fusion protein and complexes that it forms with monoclonal antibodies. Virology 271122-131. - PubMed
-
- Chambers, P., C. R. Pringle, and A. J. Easton. 1990. Heptad repeat sequences are located adjacent to hydrophobic regions in several types of virus fusion glycoproteins. J. Gen. Virol. 713075-3080. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

